ADXS--PSA, is an active immunotherapy utilizing live, attenuated bioengineered Listeria monocytogenes (LM) used as a vector to deliver PSA directly to antigen presenting cells (APCs). ADXS-PSA induced T cell responses not only to PSA, but also to other prostate cancer antigens that were not expressed by the LM based vector, which is indicative of antigen cascade or antigen spreading. ADXS-PSA has shown tumor growth inhibition and prolonged survival in mice models bearing tumor expressing the human PSA antigen.
Check point inhibitor monotherapy has not shown to date significant clinical activity in prostate cancer. However, there is data demonstrating synergistic activity of the combination of ADXS-PSA with a PD-1 blocking antibody in a murine model.
Keynote 046 is an ongoing phase 1 / 2 trial (NCT02325557) assessing the safety, tolerability and efficacy of ADXS-PSA administered as monotherapy and in combination with pembrolizumab to patients with highly refractory MCRPC. The authors now present interim results including safety and preliminary clinical activity. A total of 50 patients were treated in both part A (n=13 patients) and part B (n=37 patients).
This open label multicenter, nonrandomized study is divided to 2 parts as demonstrated in figure 1.
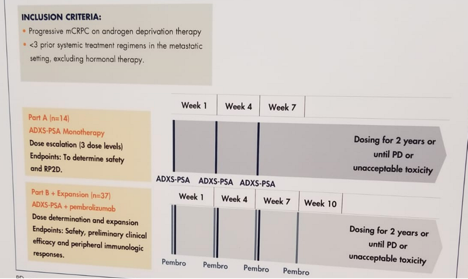
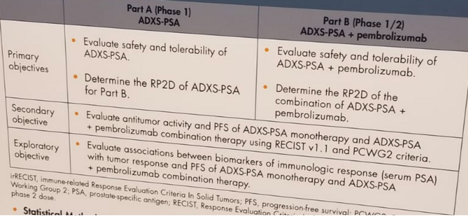
Part A monotherapy has been completed. Part B of combination therapy is currently ongoing. Summarized study objectives are shown in figure 2.
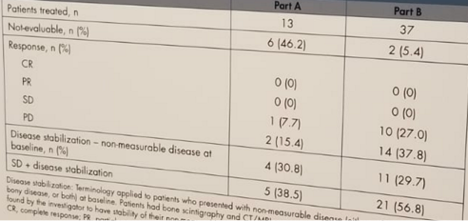
In part A and part B 35.7% and 67.6% of the patients respectively, discontinued treatment due to disease progression. To date, five patients remain on treatment in part B. When assessing the safety, 98% experienced any grade treatment related adverse events. Most of these events were grade 1-2 including chills/rigors, fever, hypotension, nausea and fatigue. The combination therapy did not increase the rate of adverse events. Serious adverse events were reported in 10 patients, 2 in part A and 8 in part B. Best overall response using RECIST 1.1 criteria are shown in figure 3.
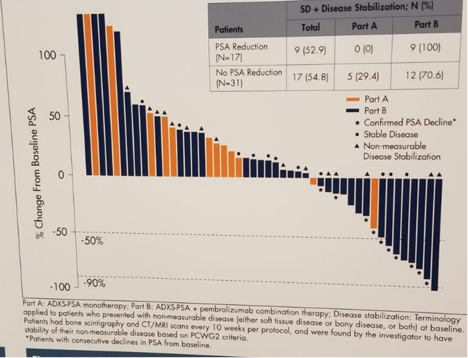
Maximal change in PSA at any time since treatment initiation is shown in figure 4.
The authors concluded that the combination treatment of ADXS-PSA and pembrolizumab appeared safe and tolerable in these highly refractory patients, with mostly grade 1-2 treatment related adverse events. The combination therapy resulted in the longest overall survival thus far. Lastly, correlative immunologic analyses and overall survival for the combination therapy patients are still undergoing.
Presented by: Mark N. Stein, Columbia University Medical Center, New York, NY
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter:@GoldbergHanan at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA


