In the immunotherapy era, KEYNOTE-564 was the first trial showing a disease-free survival (DFS) benefit of adjuvant pembrolizumab in comparison to placebo for patients with clear-cell RCC (ccRCC) who met the protocol-defined criteria for high risk of recurrence (stage T2 with nuclear grade 4 or sarcomatoid differentiation, stage >T3, regional lymph-node metastasis, or stage M1 with no evidence of disease).2 The DFS benefit was confirmed by an updated analysis at 30-mo follow-up (hazard ratio 0.63, 95% confidence interval 0.50-0.80).3 These results led to the approval of adjuvant pembrolizumab by the US Food and Drug Administration and the European Medicines Agency.
Recently, the overall survival results from the KEYNOTE-564 study were presented at the ASCO GU meeting 2024.4
The European Association of Urology (EAU) guidelines include a weak recommendation for offering adjuvant pembrolizumab to ccRCC patients with a recurrence risk as defined in the KEYNOTE-564 trial.1 Nonetheless, surgery alone can cure patients with nonmetastatic RCC and the EAU guidelines strongly recommend informing patients about the potential risk of overtreatment and immune-related side effects,5 and discussing the contradictory results from other adjuvant immune checkpoint inhibitor (ICI) trials to facilitate shared decision-making.1 Indeed, the ultimate value of adjuvant pembrolizumab for RCC has been questioned.6-8
Arguably, patients who might benefit the most from adjuvant pembrolizumab are those whose probability of RCC recurrence outweighs their probability of dying from other causes over a reasonable timeframe after surgery.
To assess the potential impact of this hypothesis on ‘‘eligibility’’ for adjuvant pembrolizumab among patients with ccRCC meeting the KEYNOTE-564 study criteria, we applied the risk-adapted surveillance approach proposed by Stewart-Merrill et al to a multicentre cohort of patients who underwent surgery for nonmetastatic RCC.9 This approach offers age-, Charlson comorbidity index (CCI)-, stage-, and relapse location–specific estimates of RCC follow-up durations after nephrectomy, with stopping of follow-up warranted when the estimated risk of other-cause mortality (OCM) outweighs the estimated risk of RCC recurrence.9
We hypothesised that patients for whom follow-up could be stopped after a ‘‘prespecified’’ time period (eg, 2, 5, 10, or 20 yr) might derive less benefit from adjuvant pembrolizumab (despite eligibility according to the trial criteria) given their lower probability of living long enough for a DFS (and potentially OS) benefit from adjuvant treatment.
We queried our prospectively collected database of consecutive patients with nonmetastatic (cT1–4 N0–1 M0) renal masses who underwent partial or radical nephrectomy between 2015 and 2021 at four referral academic centres to identify all patients with ccRCC meeting the KEYNOTE-564 protocol-defined criteria. We stratified these patients using the risk-adapted model proposed by Stewart-Merrill et al. (Fig. 1).9
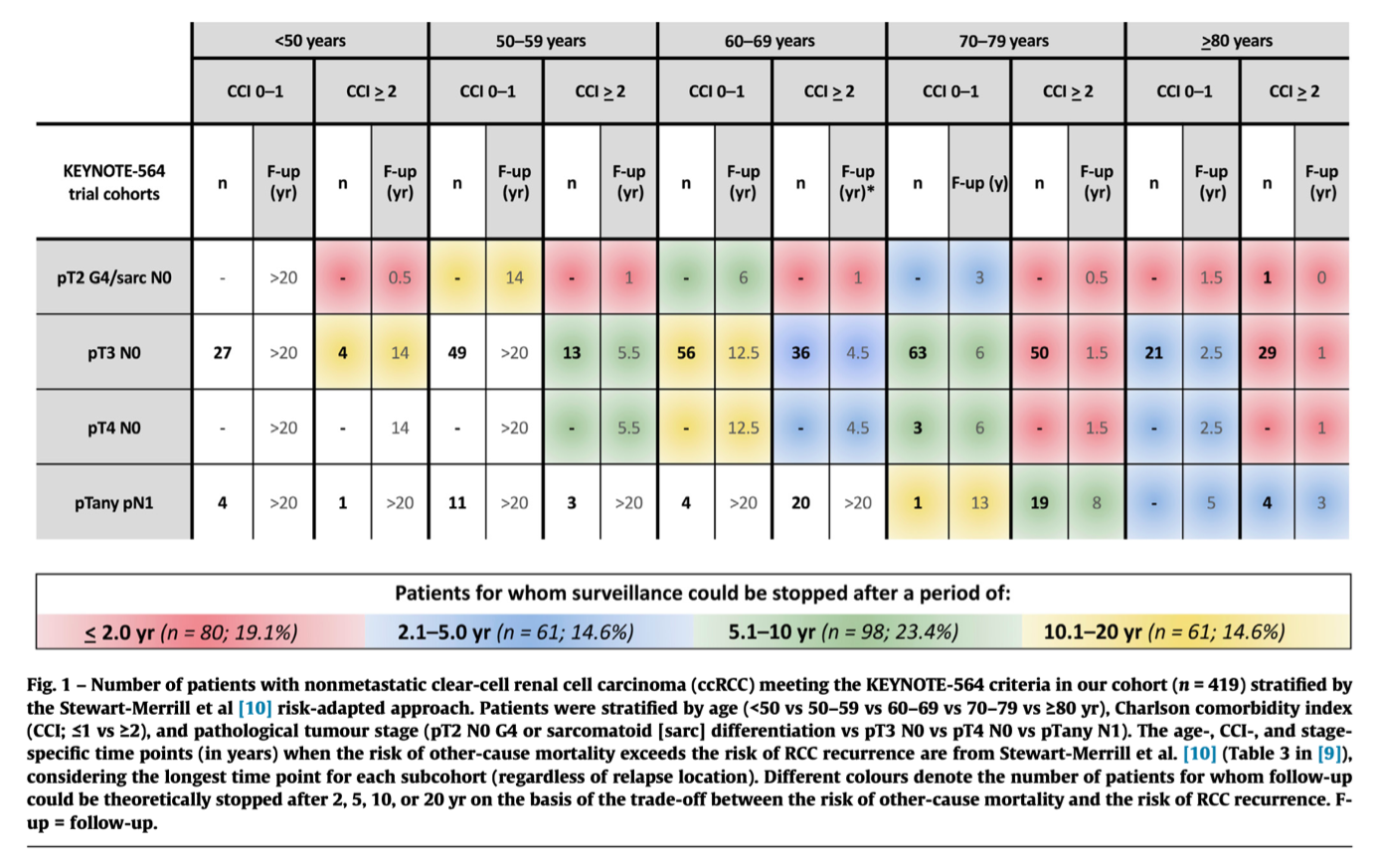
We then explored the proportion of patients whose follow-up could theoretically be stopped at 2, 5, 10, or 20 yr, for whom ‘‘eligibility’’ for adjuvant pembrolizumab might be more controversial, as offering adjuvant pembrolizumab might theoretically be of value only for patients whose recommended follow-up is reasonably long.
Overall, we identified 1745 patients diagnosed with ccRCC, of whom 419 (24%) met the KEYNOTE-564 criteria and were included in our analytical cohort. Most of these patients (83.1%) had pT3N0 ccRCC.
According to our analysis, follow-up could have been stopped:
- after <2.0 yr for 80/419 (19.1%)
- after 2.1–5.0 yr for 61/419 (14.6%)
- after 5.1–10.0 yr for 98/419 (23.4%), and
- after 10.1–20.0 yr for 61/419 (14.6%) patients.
Based on these data, the proportion of patients ‘‘not eligible’’ for adjuvant pembrolizumab because of higher probability of OCM than of RCC recurrence would be 81%, 66%, 43%, and 29% for ‘‘recommended’’ follow-up of >2.0, >5, >10, and >20 yr, respectively (Fig. 2).

Our study provides insights to support shared decision-making regarding eligibility for adjuvant pembrolizumab for patients with nonmetastatic ccRCC at higher risk of recurrence.
Our data suggest that ‘‘eligibility’’ for adjuvant pembrolizumab could theoretically be tailored to the individual patient’s competing risks of RCC recurrence and OCM.9 Using this strategy, multidisciplinary tumour boards could reserve the indication for adjuvant pembrolizumab for patients whose probability of ccRCC recurrence outweighs their probability of OCM over a reasonable timeframe (eg, 5 or 10 yr after surgery), which could potentially lead to a reduction in overtreatment, better use of resources, and better value for patients.
Considering the prevalence of elderly and/or comorbid patients with ccRCC in real-life practice, this strategy could represent a first step towards individualised selection of candidates for adjuvant pembrolizumab according to a value-oriented paradigm.10
While our study is not devoid of limitations, to the best of our knowledge, this is the first study aiming to support decision-making regarding eligibility for adjuvant pembrolizumab with a focus on patient-related factors beyond currently available prognostic scores for the risk of RCC recurrence.1 While artificial intelligence and novel biomarkers could help in refining current prognostic models for ccRCC, further studies are needed to individualise the eligibility criteria for adjuvant pembrolizumab and prospectively assess the impact on oncological outcomes, patient-reported outcome measures, and costs for patients meeting the inclusion criteria of randomised clinical trials.11
Written by: Riccardo Campi, MD, PhD, FEBU, Unit of Urological Robotic Surgery and Renal Transplantation, Careggi Hospital, University of Florence, Florence, Italy; Department of Experimental and Clinical Medicine, University of Florence, Florence, Italy; Young Academic Urologists Renal Cancer Working Group, Arnhem, The Netherlands
References:
- Ljungberg B, Albiges L, Bedke J, et al. EAU guidelines on renal cell carcinoma. Arnhem, The Netherlands: European Association of Urology; 2023.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant pembrolizumab after nephrectomy in renal-cell carcinoma. N Engl J Med 2021;385:683–94.
- Powles T, Tomczak P, Park SH, et al. Pembrolizumab versus placebo as post-nephrectomy adjuvant therapy for clear cell renal cell carcinoma (KEYNOTE-564): 30-month follow-up analysis of a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2022;23:1133–44.
- ASCO GU 2024: Overall Survival Results from the Phase 3 KEYNOTE-564 Study of Adjuvant Pembrolizumab vs Placebo for the Treatment of Clear Cell RCC. (n.d.). Www.urotoday.com.
- Bedke J, Bex A. TNM-based risk eligibility for adjuvant trials in renal cell carcinoma. Lancet 2023;402:1018–9
- Tannock IF, Goldstein DA, Ofer J, et al. Evaluating trials of adjuvant therapy: is there benefit for people with resected renal cancer? J Clin Oncol 2023;41:2713–7.
- Marchioni M, Amparore D, Marandino L, et al. Is adjuvant immunotherapy worth for all patients with clear-cell renal cell carcinoma at high risk of recurrence? Eur Urol Open Sci 2022;46:39–42.
- Sharma V, Wymer KM, Joyce DD, et al. Cost-effectiveness of adjuvant pembrolizumab after nephrectomy for high-risk renal cell carcinoma: insights for patient selection from a Markov model. J Urol 2023;209:89–98.
- Stewart-Merrill SB, Thompson RH, Boorjian SA, et al. Oncologic surveillance after surgical resection for renal cell carcinoma: a novel risk-based approach. J Clin Oncol 2015;33:4151–7.
- Reitblat C, Bain PA, Porter ME, et al. Value-based healthcare in urology: a collaborative review. Eur Urol 2021;79:571–85.
- Khene ZE, Bigot P, Doumerc N, et al. Application of machine learning models to predict recurrence after surgical resection of nonmetastatic renal cell carcinoma. Eur Urol Oncol 2023;6:323–30.


