Prostate cancer accounts for a large number of cancer-related deaths. When the tumor growth continues even after surgical castration (castration-resistant prostate cancer), overproduction and sensitivity to androgens by cancerous cells can be used for chemotherapeutic intervention. One promising target has been the production of dehydroepiandrosterone (DHEA), a precursor to androgen production, by the enzyme cytochrome P450 CYP17A1. Current treatment options include a drug called abiraterone. However, like many other anti-cancer drugs, abiraterone is not very specific and has strong side effects. Humans also stop cortisol production upon abiraterone injection and treatment with prednisone is required to combat the adverse side effects. Moreover, the life extension gained from abiraterone treatment is only a few months. Previous work from the laboratory of Amit Pandey has established that abiraterone also inhibits cortisol production in humans by inhibition of another cytochrome P450 enzyme called CYP21A2.
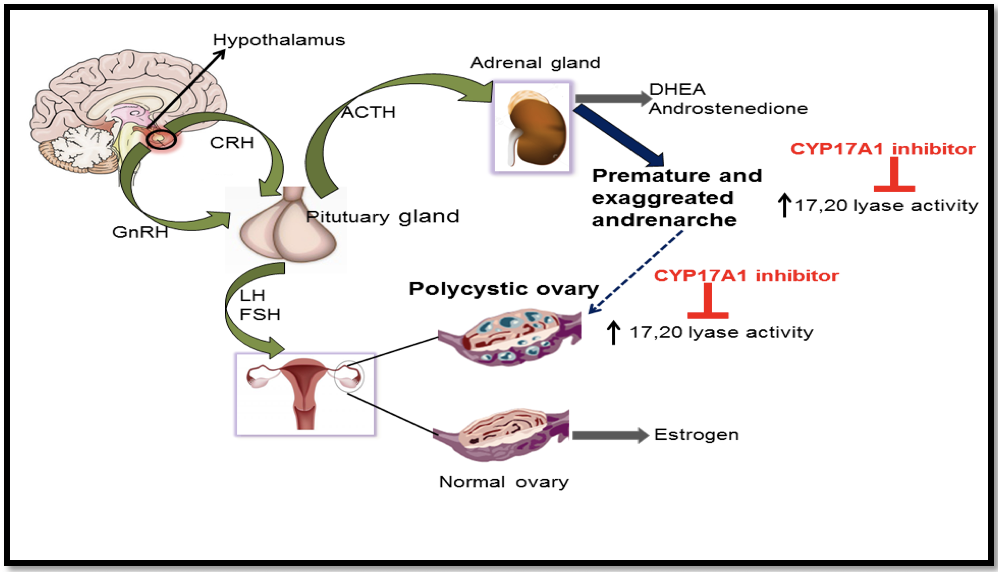
An overview of androgen production in humans and their targeting by drugs. From Udhane et al Biochem Biophys Res Commun. 2016 Sep 2;477(4):1005-1010.
A research team from the Division of Pediatric Endocrinology, Bern University Hospital, that is also affiliated with the Department for Biomedical Research of the University of Bern, in collaboration with long-term colleagues from Vall d’Hebron Research Institute in Barcelona has found a novel mutation in humans that illustrates how steroids and drugs specifically bind to cytochrome P450 CYP17A1. Their findings could lead to a better drug for the treatment of castration-resistant prostate cancer. The results of this study have been published in the Open-Access Journal "Pharmaceuticals".
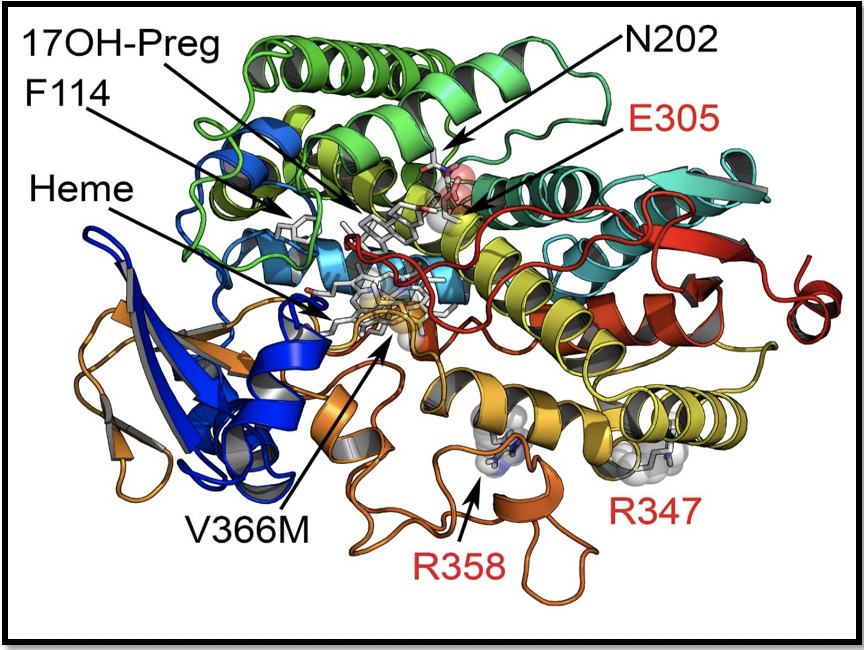
Location of novel V366M mutation at the active site of CYP17A1. From Pharmaceuticals, 2018 11, no. 2: 37
Observe and learn from experiments of Nature!
The CYP17A1 has been a hot topic of research over the past four decades and carries out two unique reactions in the metabolism of steroids. The first activity called the 17α-hydroxylase activity is essential for the production of 17OH-pregnenolone and 17OH-progesterone which are precursors of cortisol, and the second activity known as the 17,20 lyase reaction is required for the generation of the precursor of sex steroids, dehydroepiandrosterone (DHEA). These two activities of the CYP17A1 determine the type of steroid hormone synthesized in different cells; if the CYP17A1 is totally absent, mineralocorticoids are produced, if only the 17α-hydroxylase activity of the CYP17A1 is present, glucocorticoids are made; and if both the 17α-hydroxylase and the 17,20 lyase activities of the CYP17A1 are present, sex steroid precursors are generated. Overproduction of androgens by the specific activation of CYP17A1-17,20 lyase activity has been implicated in the pathogenesis of the polycystic ovary syndrome. In the late 1990s study of human mutations in this protein by Walter L Miller at the University of California San Francisco (UCSF), revealed some point mutations that caused isolated 17,20 lyase deficiency, where only the production of DHEA was disrupted. However, these mutations were not in the place where steroids bind inside the CYP17A1 and search for further clues into the unique activities this enzyme continued.
Amit Pandey in Bern has been studying steroid production in humans for many years. He had recently been investigating whether anti-prostate cancer drugs were truly specific. In 2015 Dr. Sameer Udhane working in Pandey laboratory carried out sophisticated steroid measurements using radioactive steroids and advanced detection techniques which suggested that along with CYP17A1, the drug abiraterone may also impact multiple metabolic pathways. After publication of this work in 2016, Dr. Jana Malikova took up this work further and investigated the details of this off-target effect of abiraterone on CYP21A2. In collaboration with Prof. Rita Bernhardt and Dr. Simone Brixius-Anderko at the University of Saarland in Germany, the Pandey laboratory established molecular level details of abiraterone binding to CYP21A2 and provided direct evidence of cortisol suppression by abiraterone. While all these studies were being carried out, collaborators in Barcelona, led by Dr. Laura Audi, identified a patient that potentially had loss of 17,20 lyase activity. Dr. Mónica Fernández-Cancio and Dr. Nuria Camats in Barcelona then carried out genetic and biochemical analysis and reported a novel mutation in the CYP17A1 gene.
When the molecular biology experiments were started in Bern, Pandey immediately realized that this is not some ordinary mutation but is located at the exact site in the CYP17A1 where all the steroids bind to the protein and get converted to different metabolites. For the first time, a mutation at the active site of CYP17A1 had been found. Laboratories of Christa E Flück and Amit Pandey in Bern and Audi laboratory in Barcelona then set forward to examine the minute details of the effects of this mutation to see if it gives any clues towards specific nature of 17,20 lyase reaction of CYP17A1, and therefore, could provide leads for design of better and specific drugs against prostate cancer and polycystic ovary syndrome. When researchers in Bern and Barcelona performed hormonal and biochemical measurements, Pandey assigned computational chemist Dr. Adam Zalewski from his laboratory to use computational Molecular Dynamics simulations to learn more about how drug binding and action in CYP17A1 can be affected by this mutation. Dr. Zalewski ran multiple scenarios of simulations using advanced quantum mechanics and measured computationally the binding of different steroids and drugs to natural and mutated CYP17A1 and reported that he can see a change in distance between steroids and the center of CYP17A1 after the mutation. These changes meant that only the production of DHEA was being affected and for the first time, spatial requirements for 17,20 lyase reaction can be understood. Biochemical studies in the laboratory found that production of DHEA by the CYP17A1 protein with the mutation was impaired. Further confirmation came from the analysis of urine steroid profile of the patient by Dr. Bernhard Dick at the Department of Nephrology and Hypertension of Bern University Hospital, who noticed that androgen precursors were negligible in the urine of this patient with the CYP17A1 mutation. After compiling this complex report with input from colleagues with expertise in different areas of medicine and biology, the finding was recently published.
These results can be exploited for development of novel therapeutic agents as Pandey explains: by understanding the location of drug binding to CYP17A1 and small details of steroid binding and metabolism by CYP17A1, we could design better inhibitors that fill up the CYP17A1 active site tightly and only affect the 17,20 lyase activity, thereby avoiding the undesirable effects that come from inhibition of 17α-hydroxylase activity of CYP17A1. A more specific inhibitor of CYP17A1 17,20 lyase activity could potentially be without off-target effects on CYP21A2 and CYP19A1 (aromatase, which converts androgens to estrogens) and other related proteins. Pandey hopes that knowledge from this study will lead to the development of new and specific drugs for the treatment of prostate cancer and polycystic ovary syndrome.
An example of International teamwork in translational biomedical research
This research is not only interesting for potential towards drug development and understanding the complexities of metabolism in prostate cancer and polycystic ovary syndrome at the molecular level, but also because of truly translational nature of the bedside to benchwork. This could not have been achieved by either team in Bern or Barcelona alone, Amit Pandey emphasizes. Groups in Barcelona and Bern have strong expertise in different areas; The Audi group in Barcelona provided their decades of experience and competence in genetics and pathology and Department of Nephrology and Hypertension at University Hospital Bern, which is world leader in analysis of steroid hormones by state of the art Gas chromatography-Mass spectrometry instruments, performed urine steroid analysis that detected the absence of androgen metabolites. Laboratories of Christa E Flück and Amit Pandey at the University Children’s hospital Bern used their experience in advanced recombinant protein production, steroid molecular biology, and bioinformatics to understand the complexities of androgen production in humans. This research is a perfect example of what a close collaboration between a multidisciplinary team (pathologists, endocrinologists, geneticists, clinical chemists, structural biologists, protein biochemists and computational biologists) can achieve when focusing on joint projects.
Support from Government and Industry
Steroid research laboratory at University Children’s Hospital Bern has been active since 2004. Their research has been supported by grants from both government and industry, including the Swiss National Science Foundation (SNF), Burgergemiende Bern, Jubiläumsstiftung der Schweizerischen Mobiliar Genossenschaft, Bern University Research Foundation, Novartis Foundation for Medical-Biological Research, Roche Research Foundation and Novo Nordisk S/A. The Barcelona group has been funded by grants from the Instituto de Salud Carlos III, the Centre for Biomedical Research Network on Rare Diseases (CIBERER, Madrid), and the AGAUR—University and Research Management and Evaluation Agency, Barcelona, Spain.
Written by: PD Amit V. Pandey, MD, Pediatric Endocrinology, Diabetology, and Metabolism, University Children’s Hospital Bern and Department of Biomedical Research, University of Bern
References:
1. Original Abstract: Mónica Fernández-Cancio, Núria Camats, Christa E Flück, Adam Zalewski, Bernhard Dick, Brigitte M Frey, Raquel Monné, Núria Torán, Laura Audí and Amit V Pandey: Mechanism of the Dual Activities of Human CYP17A1 and Binding to Anti-Prostate Cancer Drug Abiraterone Revealed by a Novel V366M Mutation Causing 17,20 Lyase Deficiency. Pharmaceuticals, 2018 11, no. 2: 37
2. Jana Malikova, Simone Brixius-Anderko, Sameer S Udhane, Shaheena Parween, Bernhard Dick, Rita Bernhardt and Amit V Pandey. CYP17A1 inhibitor abiraterone, an anti-prostate cancer drug, also inhibits the 21-hydroxylase activity of CYP21A2. J Steroid Biochem Mol Biol. 2017 Nov;174:192-200. doi: 10.1016/j.jsbmb.2017.09.007
3. Sameer S Udhane, Bernhard Dick, Qingzhong Hu, Rolf W Hartmann and Amit V Pandey AV. Specificity of anti-prostate cancer CYP17A1 inhibitors on androgen biosynthesis. Biochem Biophys Res Commun. 2016 Sep 2;477(4):1005-1010. doi: 10.1016/j.bbrc.2016.07.019


