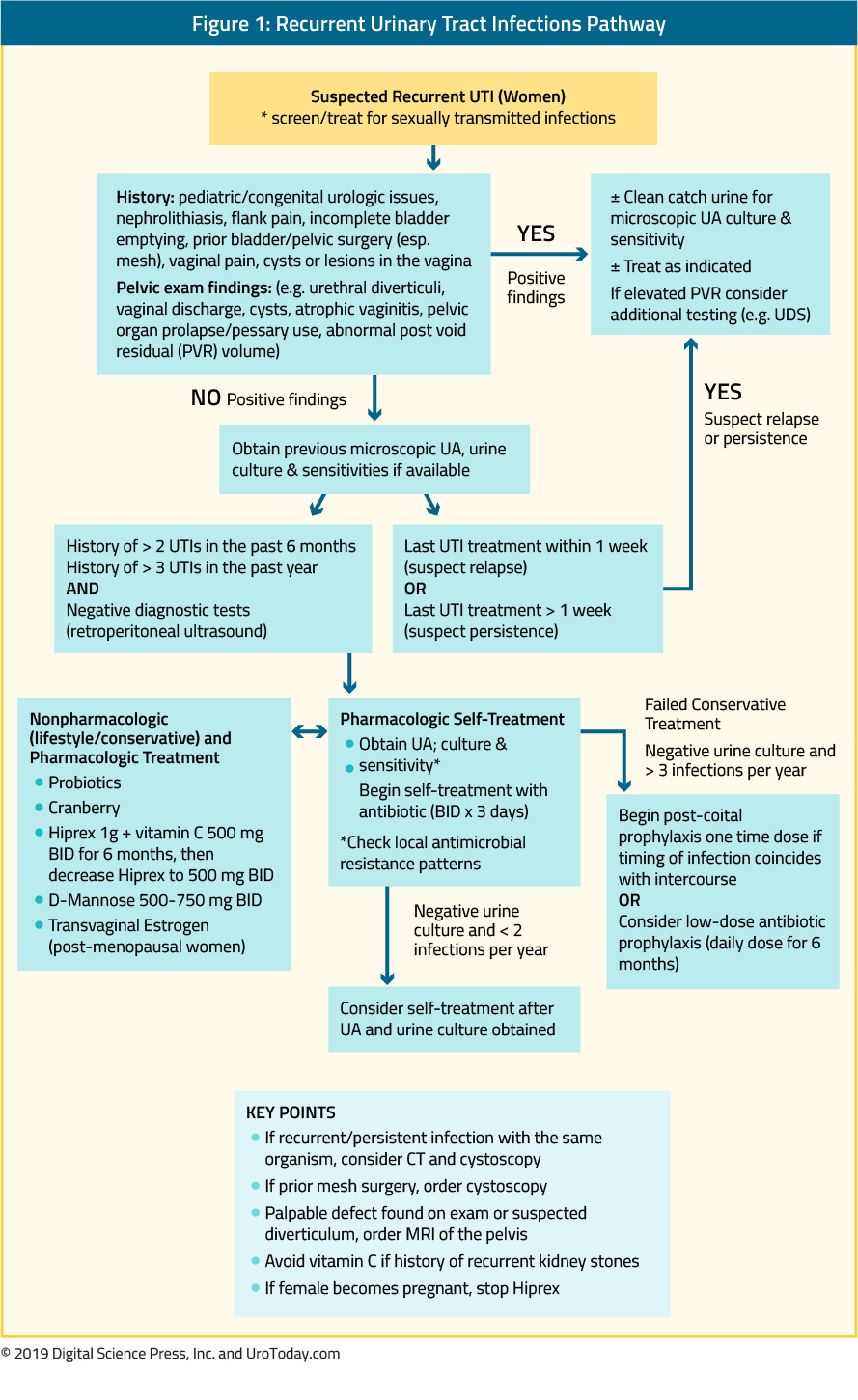
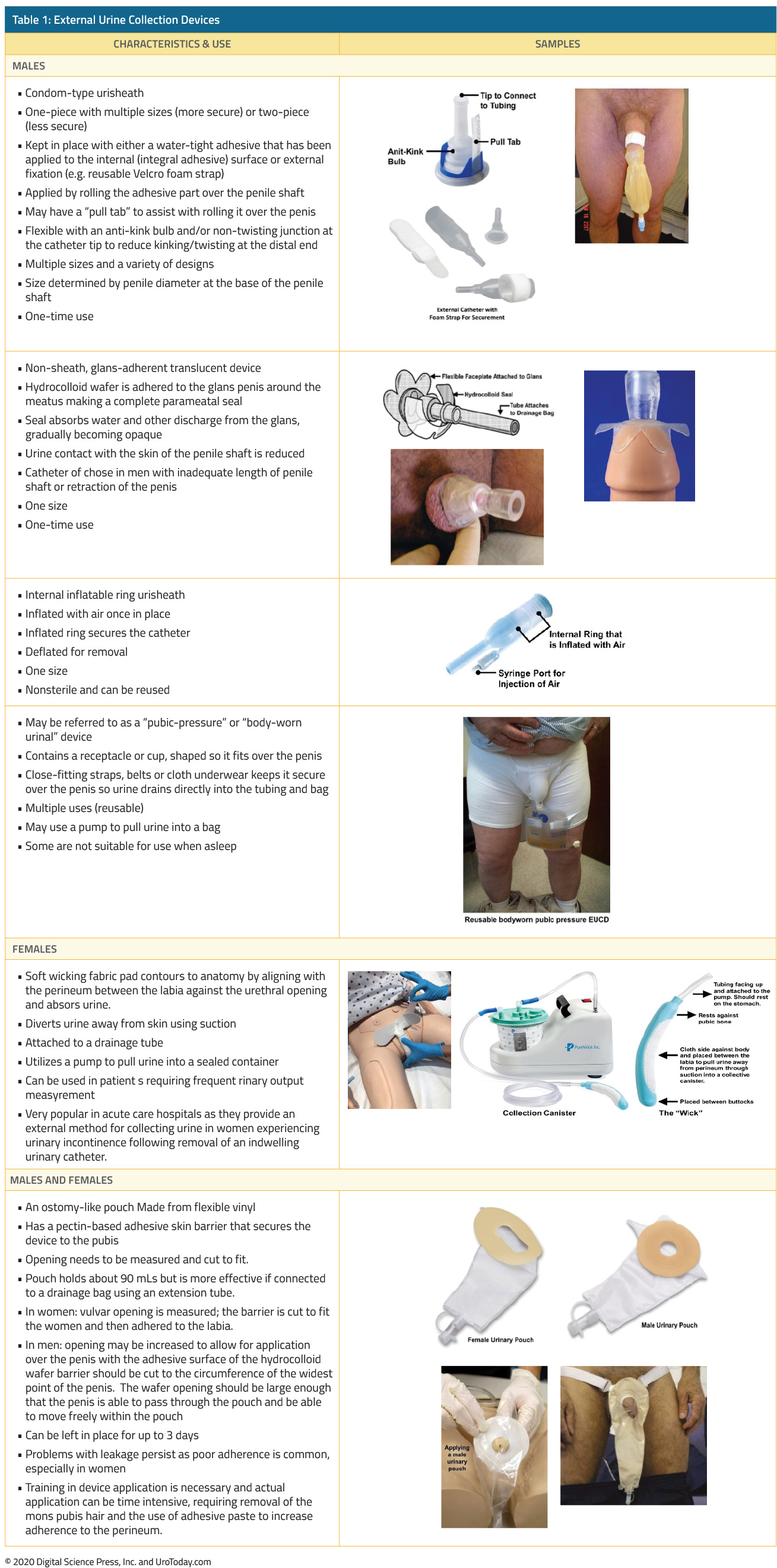
The shape and material of external urine collection devices (EUCD) have changed over the past 20 years. Historically, most EUCDs were made from latex that allowed for flexibility but also increased the risk of an allergic reaction. Latex-based sheath devices are still available but more recent ones are constructed from non-allergenic silicone. Most EUCDs are open at the distal end (tip) allowing urine to drain through attached tubing connected to a drainage bag.
There are two broad categories, those that are single-use disposable products (in-place for only one to two days), and those that are reusable for multiple times.
If the EUCD has adhesive, prior to its application, the skin should be cleansed and pubic hair at the base of the penis in men and the perineum in women should be removed. The hair should be trimmed, not shaved, because shaving causes more irritation. The EUCD can be removed by loosening the adhesive with a warm, wet cloth.
We are categorizing the types of EUCDs as follows:
Written by: Diane K. Newman, DNP, ANP-BC, FAAN, Adjunct Professor of Urology in Surgery, Research Investigator Senior, Perelman School of Medicine, Co-Director, Penn Center for Continence and Pelvic Health, Division of Urology, University of Pennsylvania, Philadelphia, Pennsylvania
April 2020