(UroToday.com) The 2024 Southern California Genitourinary Cancer Research Forum featured a prostate cancer session and a presentation by Dr. Tanya Dorff discussing key updates in prostate cancer. Dr. Dorff started her presentation by highlighting that there are new frontiers in prostate cancer, including (i) antigen targeted therapy (radioligand therapy – 177-Lu-PSMA-617, bispecific T cell engaging antibodies, and antibody drug conjugates – ABBV 969), (ii) combination immunotherapy (cabozantinib + atezolizumab in CONTACT 02), (iii) PARP inhibitors, and (iv) AR degraders (ARV 110, ARV 766, and beyond).
Dr. Dorff then discussed the VISION trial,1 which was an international, randomized, open-label phase III study that evaluated 177Lu-PSMA-617 in men with PSMA-positive mCRPC who had previously received treatment with an ARPI and one or two prior lines of taxane chemotherapy. Patients must have had PSMA-positive disease on the basis of a central review of 68Ga-PSMA-11 staging scans. PSMA positivity was defined as uptake greater in metastatic lesions than in the liver. Further, they could have no PSMA-negative metastatic lesions. Following enrollment, patients were randomized in a 2:1 fashion to receive either 177Lu-PSMA-617 plus standard of care or standard of care alone. Standard of care treatments were at the discretion of the treating investigator; however, cytotoxic chemotherapy, immunotherapy, and radium-223 were explicitly excluded. Over a median study follow-up of 20.9 months, treatment with 177Lu-PSMA-617 + standard of care significantly improved overall survival by a median of 4 months (median: 15.3 vs 11.3 months; HR: 0.62, 95% CI: 0.52 to 0.74; p < 0.001), compared to standard of care alone, in the overall cohort of all randomized patients (n=831):
Additionally, recent post-hoc analysis work suggests that stratifying patients by SUVmax is prognostic for event free probability: the higher the SUVmax quartile the better the outcomes:
Dr. Dorff notes that based on the side effect profile of 177Lu-PSMA-617 in VISION, bone marrow reserve will dictate sequences and combinations of therapy. How 177Lu-PSMA-617 compares to chemotherapy has been assessed in the TheraP trial, a phase 2 study randomizing patients to 177Lu-PSMA-617 versus cabazitaxel.2 To screen into the study, all men had both 68Ga-PSMA-11 and 18F-FDG PET/CT and were required to have high PSMA-expression (at least one site with SUVmax ≥ 20) and no sites of FDG-positive/PSMA-negative disease. All patients had progressive disease with rising PSA ≥20 ng/mL after docetaxel and 91% had received prior enzalutamide or abiraterone. After a median follow up of 13 months, 177Lu-PSMA-617 significantly improved PSA-PFS compared with cabazitaxel (HR: 0.63, 95% CI: 0.46 to 0.86) and had a much higher PSA50 rate (66% vs 37%):
Updated analysis was presented at ASCO 2022 after a median follow-up of 36 months with PFS continued to favor the 177Lu-PSMA-617 arm (HR: 0.62, 95% CI: 0.45 to 0.85). There were no significant differences in restricted mean survival time overall survival between the two arms (19.1 months in 177Lu-PSMA-617 arm versus 19.6 months in cabazitaxel arm, 95% CI for difference: -3.7 - +2.7):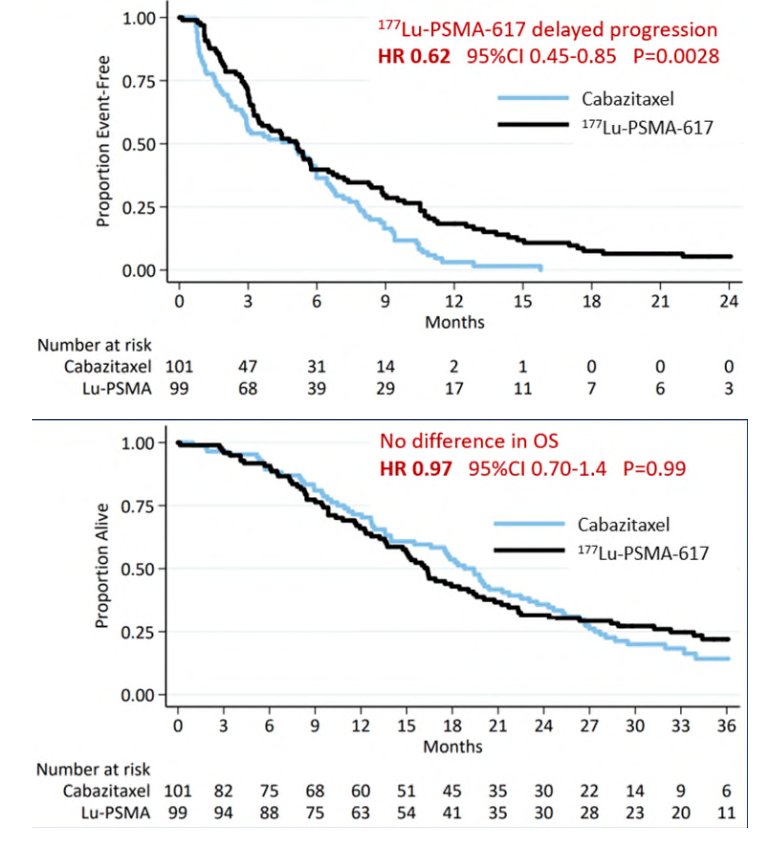
Next, the PSMAFore trial was presented at ESMO 2023 and assessed whether we can use 177Lu-PSMA-617 before chemotherapy. Eligible adults for PSMAfore had mCRPC, were candidates for ARPI change after one progression on prior ARPI, and had ≥1 PSMA positive lesions and no exclusionary PSMA negative lesions by 68Ga-PSMA-11 PET/CT. Candidates for PARP inhibition and patients with prior systemic radiotherapy (<6 months ago), immunotherapy (except sipuleucel-T), or chemotherapy (except [neo]adjuvant >12 months ago) were ineligible. Randomization was 1:1 to open-label 177Lu-PSMA-617 (7.4 GBq every 6 weeks for 6 cycles) or ARPI change (abiraterone or enzalutamide). Importantly, patients randomized to ARPI could crossover to 177Lu-PSMA-617 following centrally reviewed radiographic progression. The trial design for PSMAfore is as follows: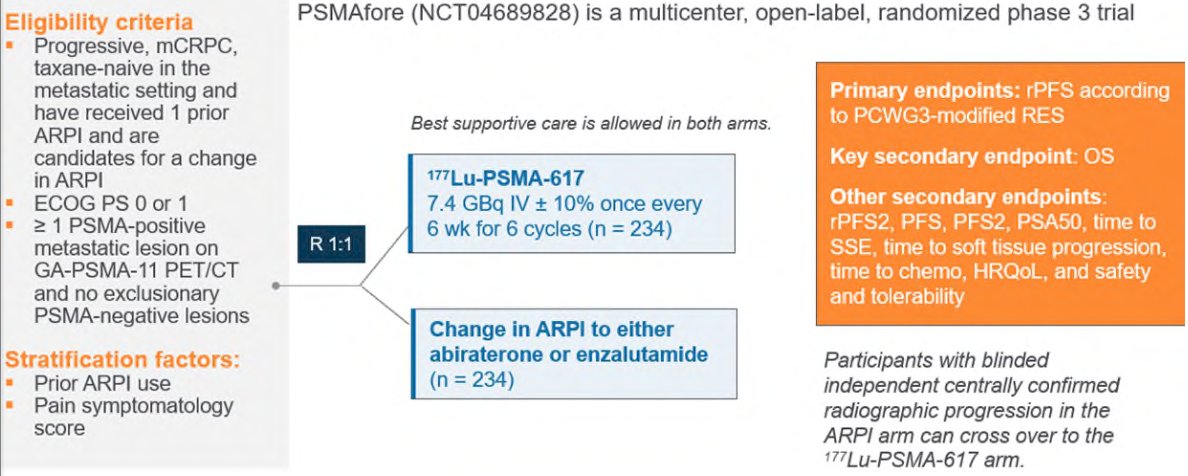
The baseline characteristics were generally well balanced between the two groups: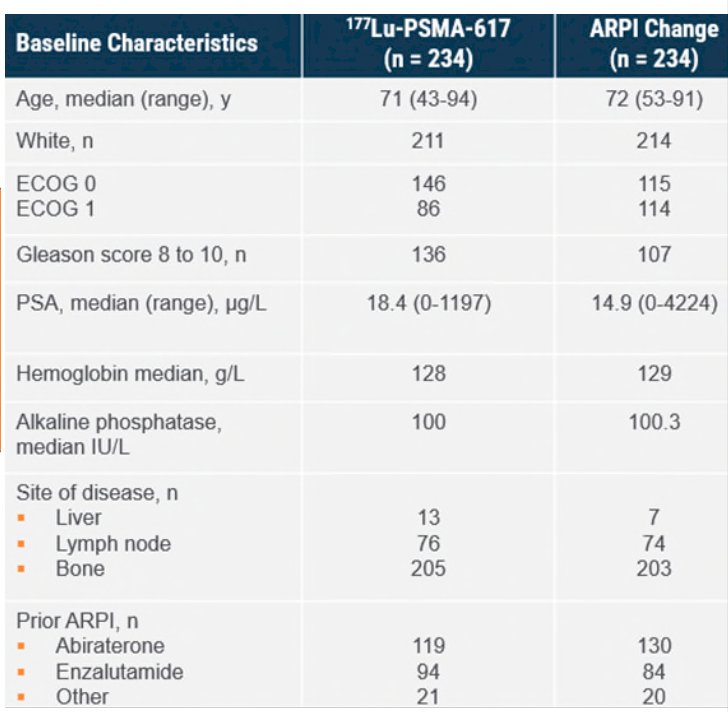
At the primary analysis (median follow-up, 7.3 months; n = 467), the primary endpoint of rPFS was met (HR 0.41, 95% CI 0.29 to 0.56), which was similar at second interim analysis (HR 0.43, 95% CI 0.33 to 0.54). Of note, the overall survival data is not mature but is trending in the opposite direction. The following is a list of ongoing trials with radioactive targeted therapy: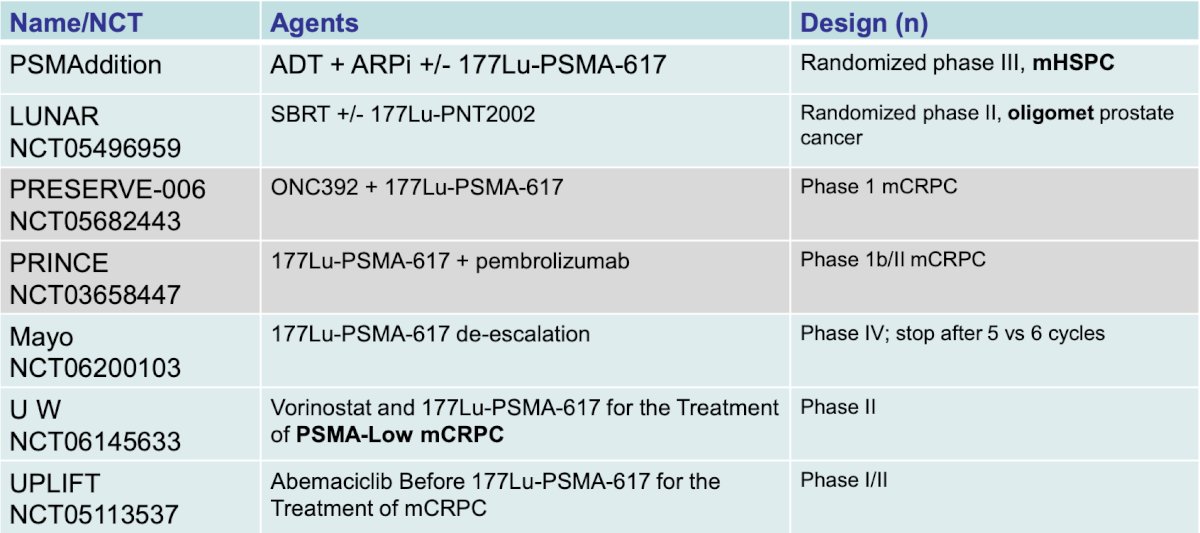
So, what’s next?
- Different particles (ie. Ac225, Pb, I131)
- Different PSMA binders
- Different protein targets (ie. hk2)
- ?Adaptive dosing
- Combinations, and in the mHSPC setting
Dr. Dorff then discussed bispecific T cell engaging antibodies for prostate cancer. AMG 160 targets PSMA 1:1 with CD3, and in a phase 1 trial showed robust response rates but limited by anti-drug antibodies. As such, toxicity has limited the ability to pursue this treatment in phase 3 trials. AMG 509 targets STEAP1 2:1 with CD3, with dose escalation data presented at ESMO 2023 showing better toxicity and efficacy, with likely movement into the phase 3 trial space. JNJ-63898081 targets PSMA 1:1 with CD3, and among 39 patients cytokine release syndrome was noted in 65% of patients, with dose limiting transaminase elevation. There were two PSA 50 responses, but no objective responses.
Dr. Dorff and colleagues on February 1, 2024, published results of a phase I study of acapatamab, a half-life extended, PSMA-targeting bispecific T-cell engager for mCRPC.3 The dose expansion phase included 56 patients of which 16.1% had grade >= 3 cytokine release syndrome and 19.6% had anemia: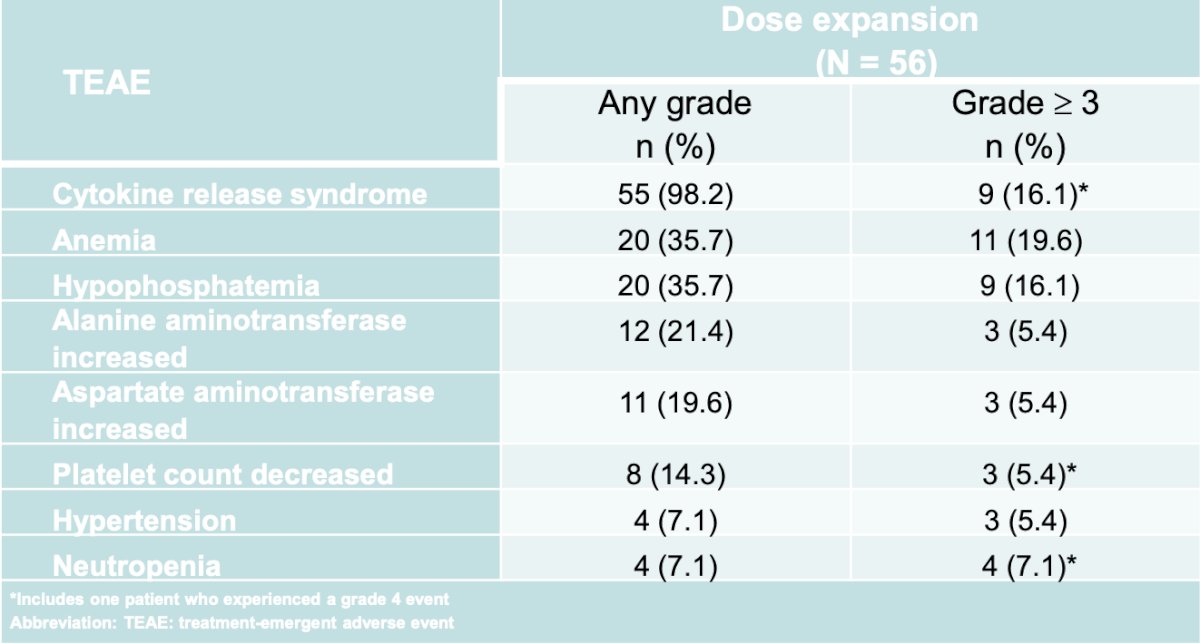
In dose expansion, confirmed PSA50 responses were seen in 30.4% of patients and radiographic partial responses in 7.4% (RECIST 1.1):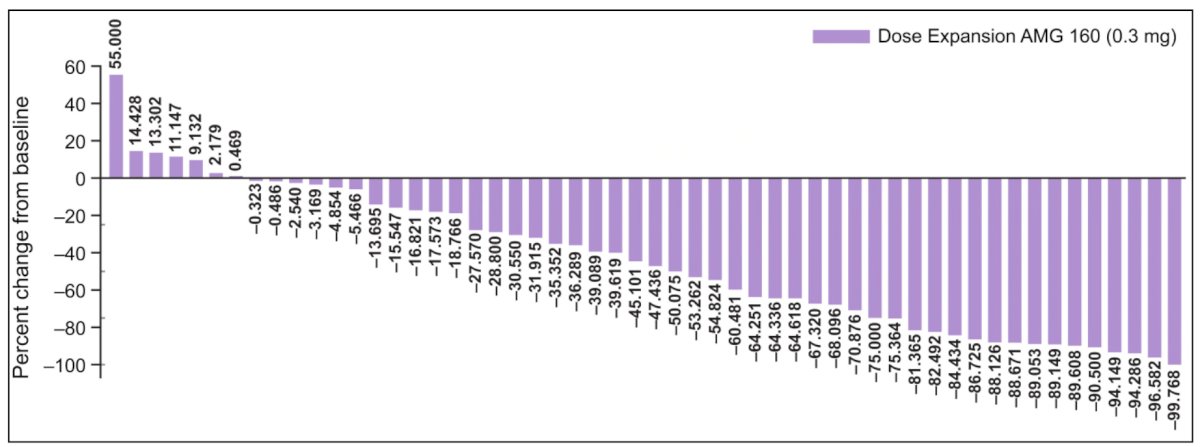
At ESMO 2023, Dr. Kelly presented the first results of the phase 1 dose escalation trial of AMG509. STEAP1 is a cell surface antigen highly expressed in prostate cancer and is associated with poor survival. Xaluritamig (AMG509) is a novel bispecific XmAb® 2+1 T-cell engager with two STEAP1 binding sites designed to facilitate T-cell–mediated lysis of STEAP1-expressing cells. Eligible patients had mCRPC refractory to prior novel hormonal therapy and 1–2 taxane regimens, ECOG 0–1, and adequate organ function. The dose exploration with step-dosing and prophylactic regimen to determine the maximum tolerated dose is as follows: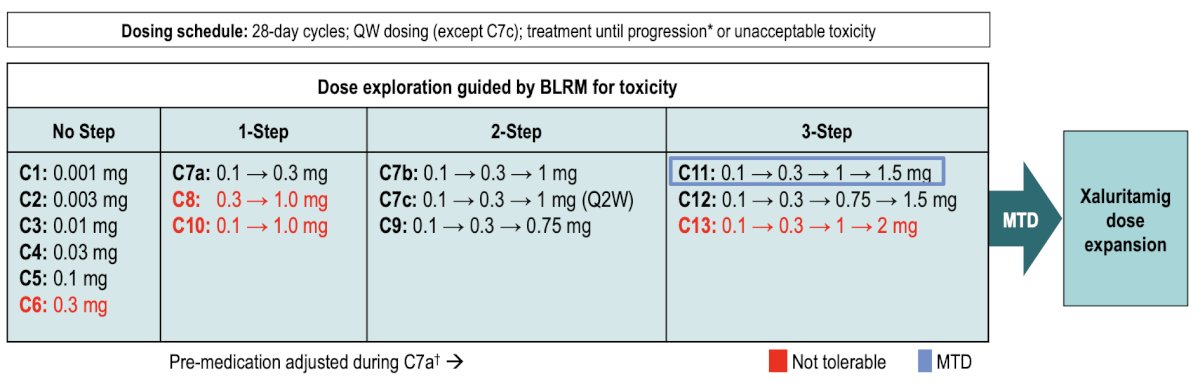
The maximum tolerated dose was identified as 1.5 mg IV weekly (3-step, D1 0.1 mg / D8 0.3 mg / D15 1.0 mg / D22+ 1.5 mg). PSA50 (≥ 50% PSA decline) responses occurred in 43 patients (49%) and PSA90 (≥ 90% PSA decline) in 24 patients (28%). PSA responses were more frequent at higher dose levels (DL7b–15) than in lower dose levels (DL1–7a):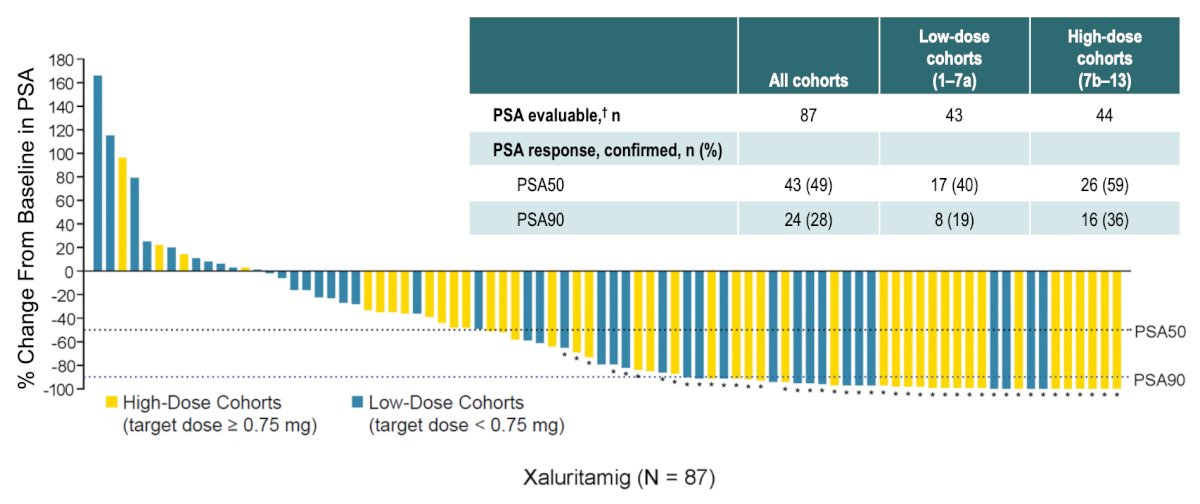
Because cabozantinib has been shown to induce favorable changes in the tumor microenvironment and regression of prostate tumors in vivo, it was subsequently combined with atezolizumab in the phase 3 CONTACT-02 trial, initially presented by Dr. Niraj Agarwal at ASCO GU 2024. Patients were randomized 1:1 to cabozantinib + atezolizumab (cabozantinib [40 mg PO daily] + atezolizumab [1200 mg IV every 3 weeks]) or control (abiraterone [1000 mg PO daily] + prednisone [5 mg PO twice daily] or enzalutamide [160 mg PO daily]) and were stratified by liver metastasis (yes/no), prior docetaxel for mCSPC (yes/no), and prior novel hormonal therapy for mCSPC, M0CRPC, or mCRPC. The trial design for CONTACT-02 is as follows: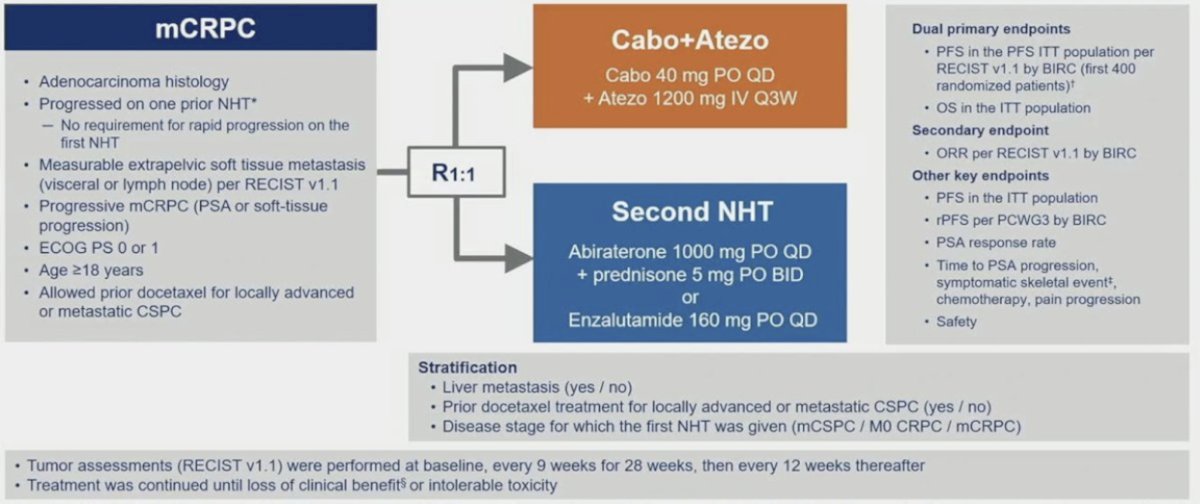
The median follow-up was 12.0 months for all randomized patients and 14.3 months for the first 400 patients. The median radiographic PFS was significantly longer with cabozantinib + atezolizumab vs control (6.3 vs 4.2 months; HR 0.65, 95% CI 0.50-0.84):
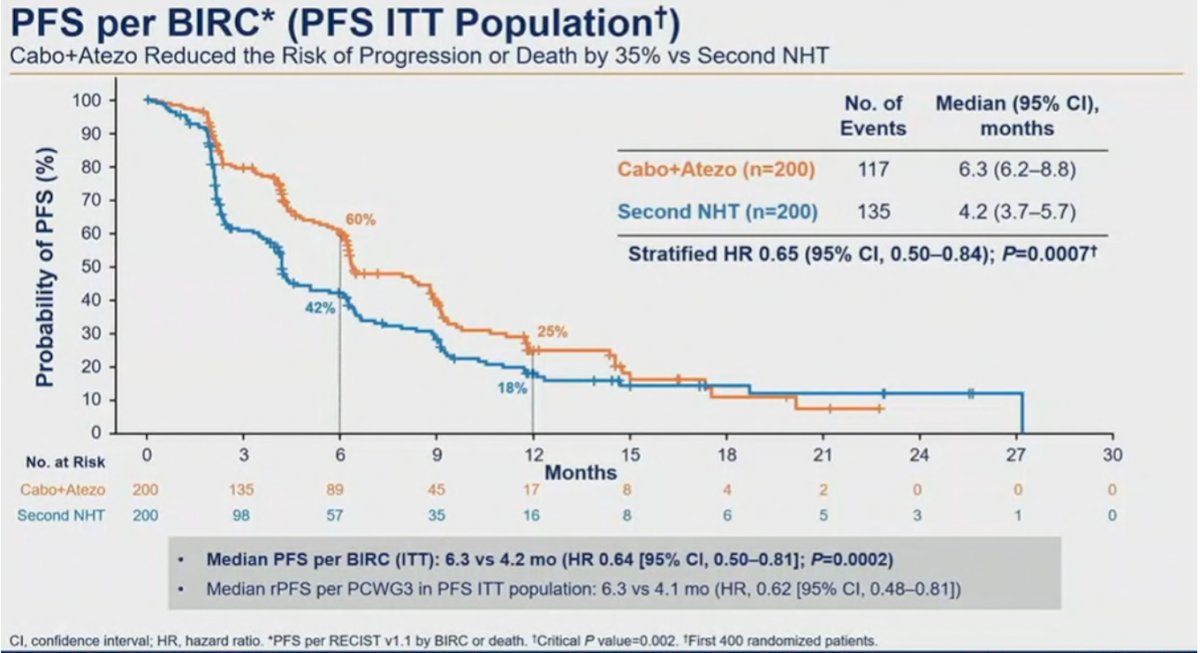
OS data is not mature yet but is trending in favor of cabozantinib + atezolizumab (HR 0.79, 95% CI 0.58-1.07). Dr. Dorff notes that one of the criticisms of this trial is the weak control arm of NHT switch (a second NHT).
Dr. Dorff then discussed PARP inhibitors, first the PROfound trial,4 which was a randomized, open-label, phase III trial evaluating efficacy and safety of olaparib versus enzalutamide or abiraterone in patients with mCRPC with alterations in any of 15 predefined genes with a direct or indirect role in homologous recombination repair whose disease had progressed on prior new hormonal agent therapy. Cohort A included patients with alterations in BRCA1, BRCA2, or ATM, while Cohort B patients included any one of 12 other homologous recombination repair alterations (BRIP1, BARD1, CDK12, CHEK1, CHEK2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D or RAD54L). Patients were randomized 2:1 to olaparib (300 mg BID) or the physician’s choice of enzalutamide (160 mg/day) or abiraterone (1000 mg/day + prednisone 5 mg BID). The primary endpoint was radiographic progression-free survival (rPFS) in Cohort A, assessed by blinded independent central review and analyzed via a stratified log-rank test. Crossover to olaparib was allowed after blinded independent central review progression: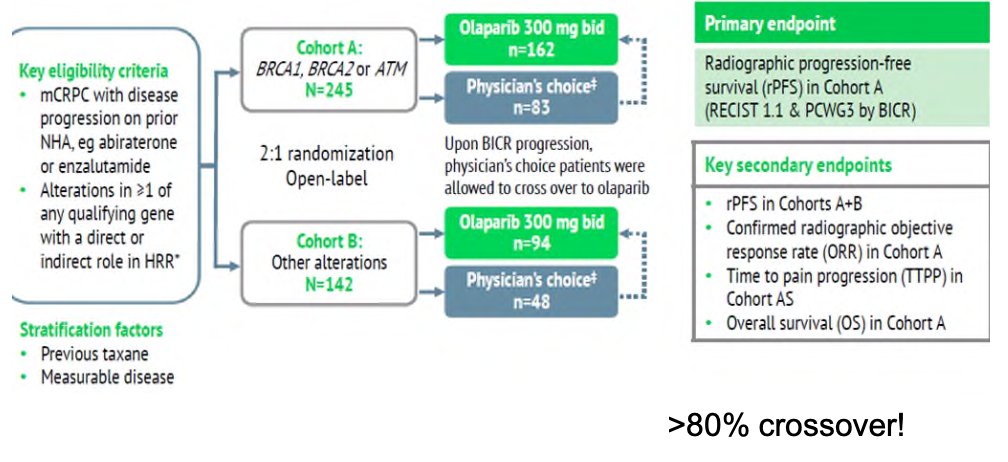
Overall survival benefits were observed in the BRCA1/2 and ATM altered cohort with median overall survival improved from 14.7 to 19.1 months (HR: 0.69, 95% CI: 0.50 – 0.97, p=0.02):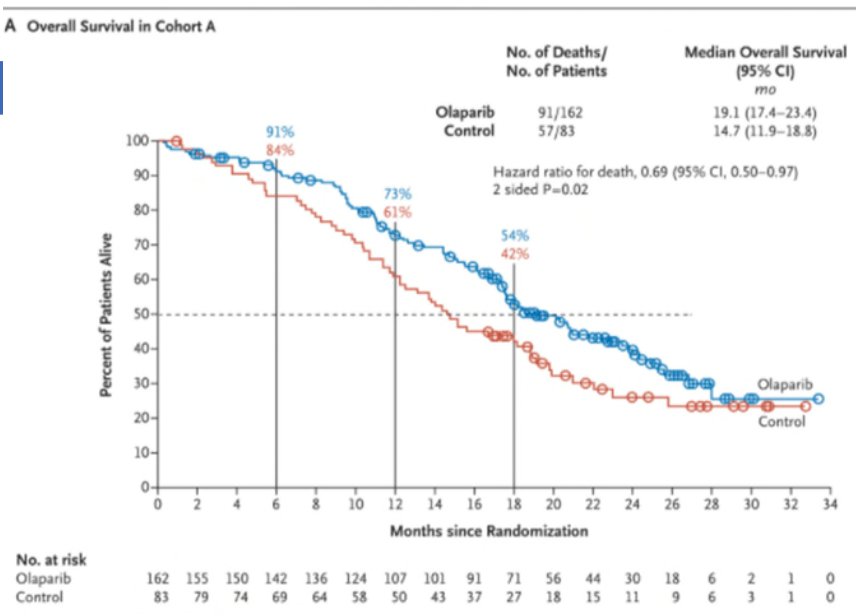
In a similar setting of the TRITON-3 trial,5 the use of rucaparib (600 mg twice daily) in mCRPC patients with BRCA1/2 or ATM alterations who had disease progression following a prior ARPI demonstrated significant improvements in imaging-based progression-free survival (median:10.2 versus 6.4 months; HR: 0.61, 95% CI: 0.47 to 0.80, p<0.001), compared to a physician’s choice control (docetaxel or an alternative ARPI). This benefit was most pronounced in the BRCA patient subgroup (median: 11.2 versus 6.4 months; HR: 0.50, 95% CI: 0.36 to 0.69, p<0.001).
TRITON2 is an international, multi-center, open-label phase II study which enrolled men with mCRPC who had disease progression following an androgen axis inhibitor and at least one taxane-based chemotherapy, and one of 13 associated homologous recombination repair gene alterations. Analysis limited to 115 patients with a BRCA alteration (median follow up 13.7 months) demonstrated an ORR of 43.5% per independent radiology review and a confirmed PSA response rate of 54.8%. Notably, ORRs were similar for patients regardless of whether a BRCA1/2 alteration was present and whether the mutation was germline or somatic. PSA response rate was higher in patients with a BRCA2 alteration.6
PROpel is the first phase III study to evaluate the combined effect of a PARP inhibitor plus an ARSI in first-line mCRPC irrespective of HRR mutation status.7 Patients were then randomized to receive full-dose abiraterone 1000 mg daily plus either placebo or full-dose olaparib 300 mg BID. The primary endpoint was investigator-assessed rPFS. OS was the key secondary endpoint. Trial schema for the phase 3 PROpel trial: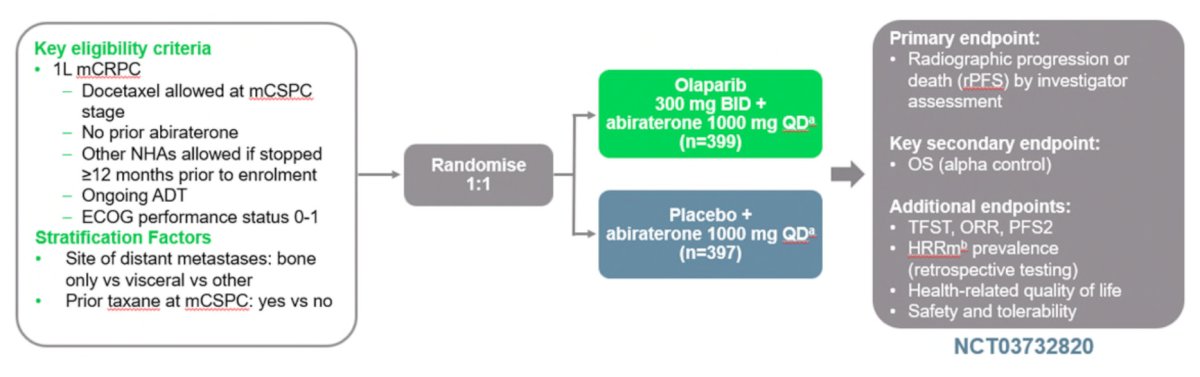
PROpel met its primary endpoint: The addition of olaparib to abiraterone resulted in a 39% reduction in risk of progression or death (HR 0.66, 95% CI 0.54 to 0.81; P < 0.0001). The addition of olaparib also improved median rPFS by 8.2 months (24.8 vs 16.6 months): 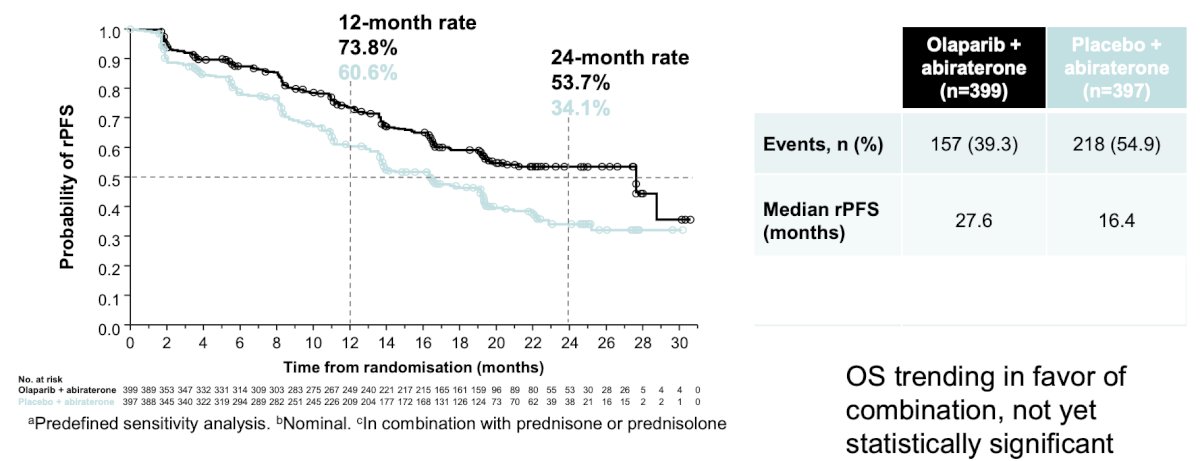
The combination of niraparib and abiraterone was evaluated in the MAGNITUDE trial, which was a randomized phase III trial of abiraterone plus niraparib or placebo as first-line therapy in patients with treatment-naïve mCRPC with and without HRR gene alterations.8 HRR positivity was defined as the presence of a deleterious alteration in one or more of the following HRR genes: ATM, BRCA1, BRCA2, BRIP1, CDK12, CHEK2, FANCA, HDAC2, or PALB2. Patients were allowed to have received up to four months of abiraterone prior to enrollment. The primary endpoint was rPFS by blinded independent central review. Secondary endpoints included OS, time to cytotoxic chemotherapy, and time to symptomatic progression. The trial design for MAGNITUDE is as follows: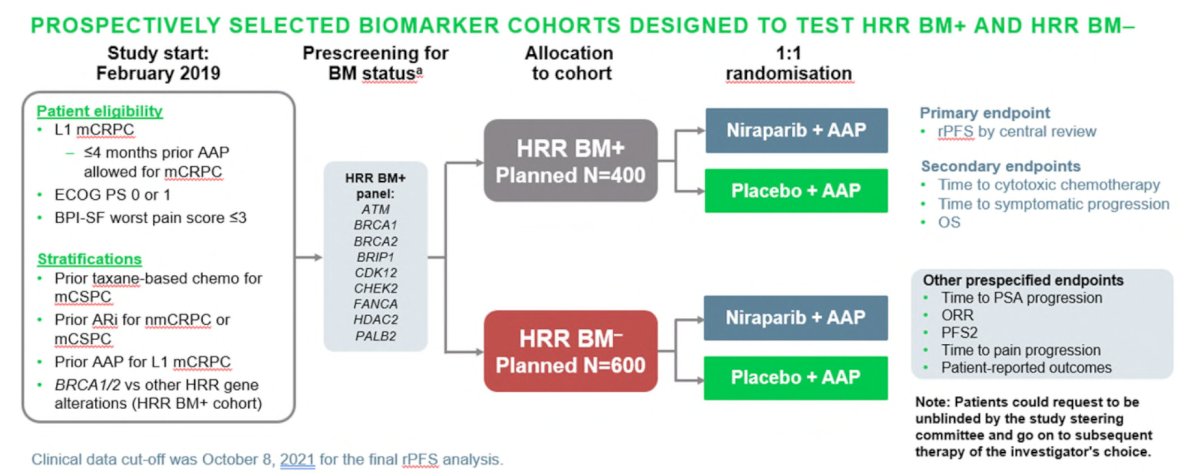
MAGNITUDE met its primary endpoint whereby in BRCA1/2-mutated patients, the addition of niraparib to abiraterone resulted in a significant improvement in rPFS by BICR, as compared with abiraterone plus placebo (HR 0.55, 95% CI 0.38 to 0.78), translating to an approximately 5.7-month improvement in median rPFS. In the overall HRR-positive cohort, the addition of niraparib to abiraterone also resulted in a significant improvement in rPFS by BICR (HR 0.73, 95% CI 0.56 to 0.96), which translated to a 2.8-month improvement in median rPFS. This rPFS improvement was, however, driven primarily by benefits in the BRCA1/2 subcohort.
Similar to the PROpel and MAGNITUDE trials, TALAPRO-2 is a phase III randomized, double-blind, placebo-controlled trial that evaluated the combination of talazoparib and enzalutamide in the first-line treatment setting for patients with mCRPC.9 Patients were randomized 1:1 to talazoparib 0.5 mg once daily (reduced dose from standard of 1.0 mg) plus enzalutamide 160 mg once daily versus placebo + enzalutamide. The primary endpoint was rPFS assessed via BICR. OS was a key secondary endpoint. At a median follow-up of 16.8-17.5 months, the combination of talazoparib + enzalutamide was associated with significant improvements in rPFS, with median rPFS not reached in the intervention arm versus 13.8 months in the placebo/enzalutamide arm (HR: 0.45, 95% CI: 0.33 – 0.81, P < 0.0001):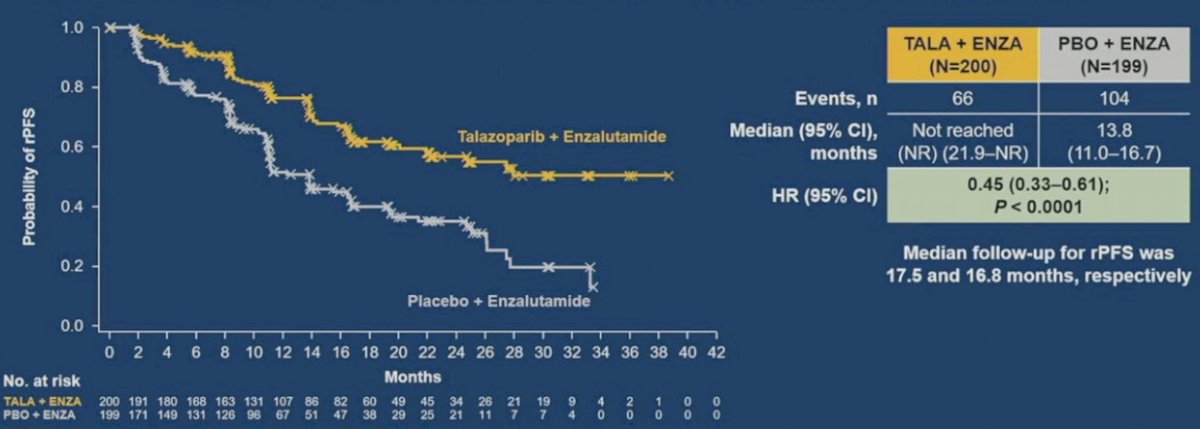
OS data remain immature (24% maturity overall). However, there appears to be an early signal for OS benefits in this HRR-mutated cohort (HR: 0.69, 95% CI, 0.46 to 1.03, p = 0.068).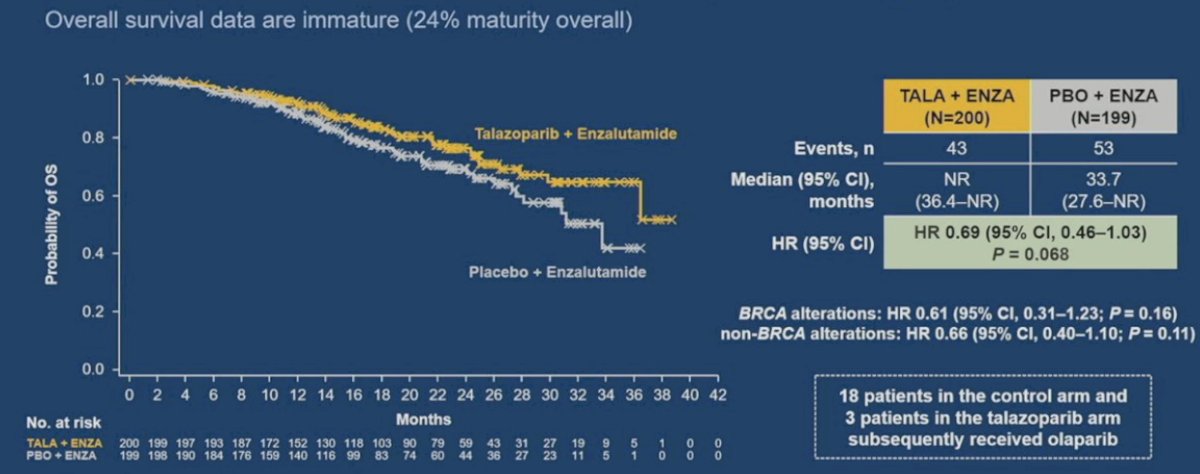
Overall, BRCA alteration is still an important biomarker to select the patients who will benefit the most.
Dr. Dorff notes that there are several notable toxicities associated with PARP inhibitors, including:
- Anemia:
- TALAPRO-1: 35% received >=1 blood transfusion
- PROfound: 21% had grade 3+ anemia
- TRITON2: 25.2% had grade 3+ anemia, 28% >=1 transfusion
- Leukopenic infection: 8% grade 3 ANC with talazoparib, 4% grade 3+ with olaparib
- Pulmonary emboli: PROfound had 4% with olaparib versus 1% with abiraterone/enzalutamide control
- Very few myelodysplastic events have occurred
The following are unanswered questions with regards to PARP inhibitors:
- How well do PARP inhibitors work in HRR+ patients aside from BRCA?
- Does it work in molecularly unselected patients with prostate cancer when combined with ARPi?
- There are certainly financial implications and barriers to treatment
- Future options include moving to the mHSPC space (for BRCA+ and ? others) and combining with radioligand therapy
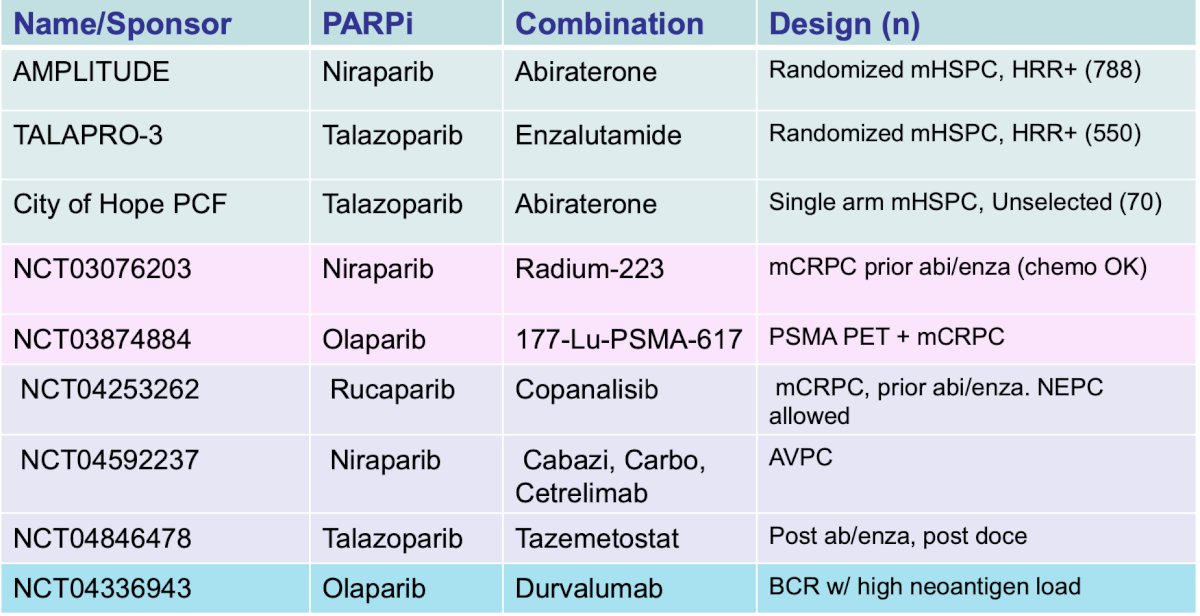
The last topic Dr. Dorff discussed was AR targeting modalities, with the following schematic diagram: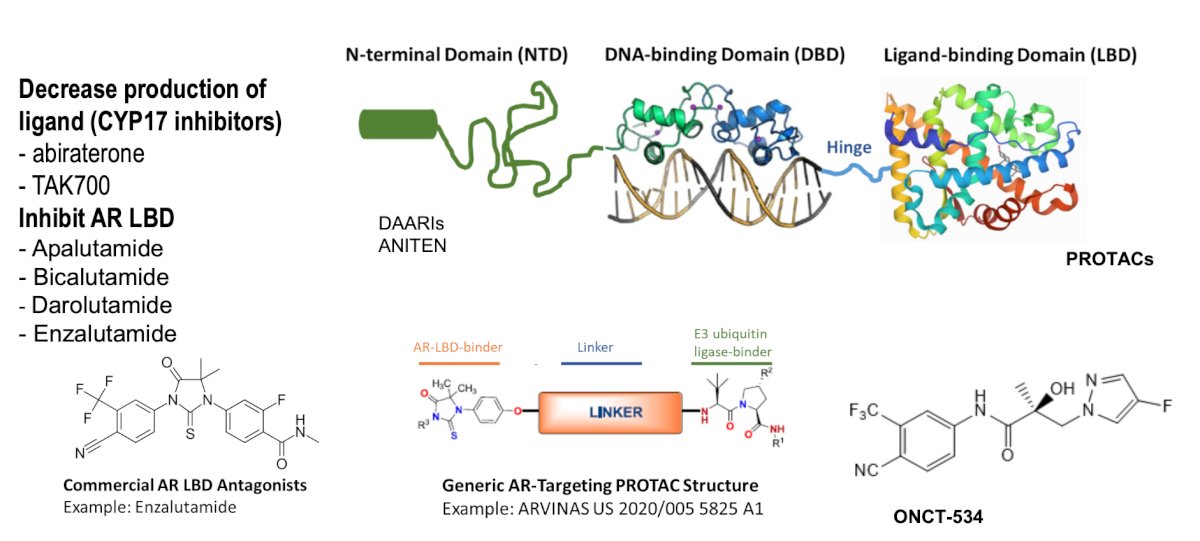
The first results of ARV-110 (bavdegalutamide) were presented at ESMO 2023 by Dr. Petrylak, which was a phase 1/2 subset analysis. Among 153 patients, PSA declines of ≥50% and ≥30%, respectively, after ≥1 month of PSA follow-up were seen in 36.4% and 50.0% of AR ligand binding domain patients (n=44) and in 53.8% and 65.4% of patients in the AR 878/875 subgroup (n=26):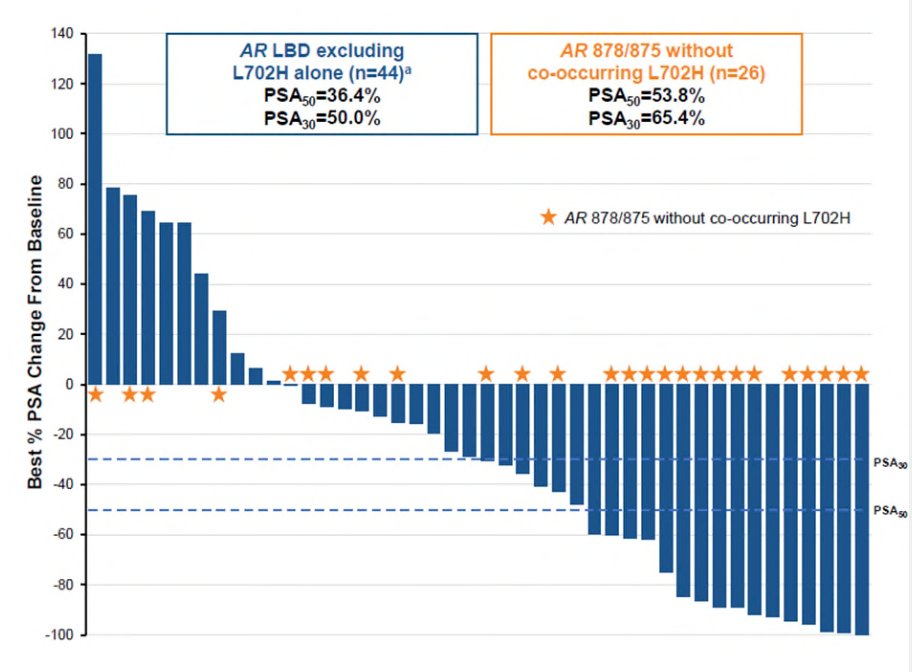
Across the phase 1/2 study, 147 (96%) of 153 patients had a treatment emergent adverse event, of which 47 (31%) had a grade 3/4 event. Overall 17 (11%) of patients had a treatment emergent adverse event that led to a dose reduction and 19 (12%) led to discontinuation. The most common treatment emergent adverse events were nausea, fatigue, and vomiting: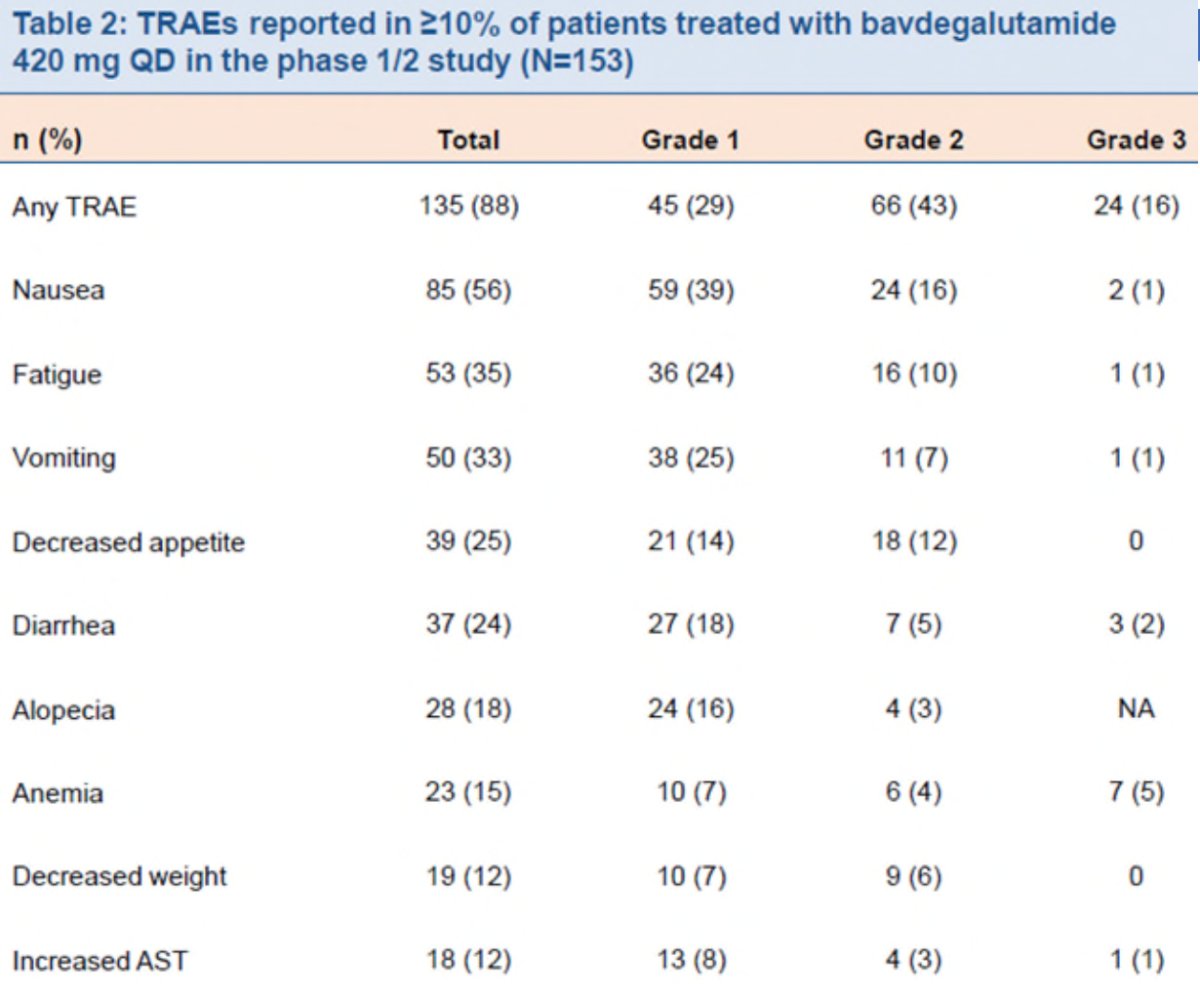
Dr. Dorff concluded her presentation discussing key updates in prostate cancer with the following summary statements:
- Radioligand therapy prolongs overall survival and has a good toxicity profile. Newer agents may increase benefit (based on different particles, binders, and targets)
- Other ways to target prostate antigens are in trials, including immunotherapy (bispecific/CAR-T), and antibody drug conjugates (cytotoxic)
- Combination of VEGF-TKI + IO may become an option, such as what has been reported in CONTACT-02 (cabozantinib + atezolizumab)
- PARP inhibitors are powerful in some mCRPC patients, and even with newer data, germline + somatic testing is important
- AR PROTAC degrades (and other novel AR targeting strategies) may be another advancement, including the ARV-766 trial open at City of Hope
Presented by: Tanya Dorff, MD, City of Hope, Duarte, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 Southern California Genitourinary Cancer Research Forum, Costa Mesa, CA, Fri, Mar 1, 2024.
References:
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021 Sep 16;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Dorff T, Horvath LG, Autio K, et al. A phase 1 study of acapatamab, a half-life extended, PSMA-targeting bispecific T-cell engager for metastatic castration-resistant prostate cancer. Clin Cancer Res. 2024 Feb 1 [Epub ahead of print].
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023;388:719-732.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772.
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomized, double-blind, phase 3 trial. Lancet Oncol. 2023 Oct;24(10):1094-1108.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023 Jun 20;41(18):3339-3351.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): A randomized, placebo-controlled, phase 3 trial. Lancet. 2023 Jul 22;402(10398):291-303.


