Other trials of anti-VEGF agents have not met their primary efficacy endpoints, and none, including sunitinib, have demonstrated an overall survival benefit. Pembrolizumab (anti-PD-1) was approved in the US and EU as adjuvant therapy based on the KEYNOTE-564 study.2 Atezolizumab (anti-PD-L1) is approved for multiple tumor types, and herein was evaluated as adjuvant therapy for patients with RCC with increased risk of recurrence. IMmotion010, a Phase III, multicenter, randomized, placebo-controlled, double-blinded trial, evaluated atezolizumab (anti-PD-L1) monotherapy as adjuvant therapy in patients with RCC and increased risk of recurrence after resection.
Key eligibility criteria for this trial included patients with RCC with a clear-cell or sarcomatoid component and who had increased risk of recurrence (T2 grade 4, T3a grade 3/4, T3b/c or T4 any grade, TxN+ any grade, or M1 resected with no evidence of disease). Patients were randomized 1:1 to atezolizumab 1200 mg IV q3w or placebo IV q3w for 16 cycles or 1 year. The trial schema for IMmotion010 is as follows:
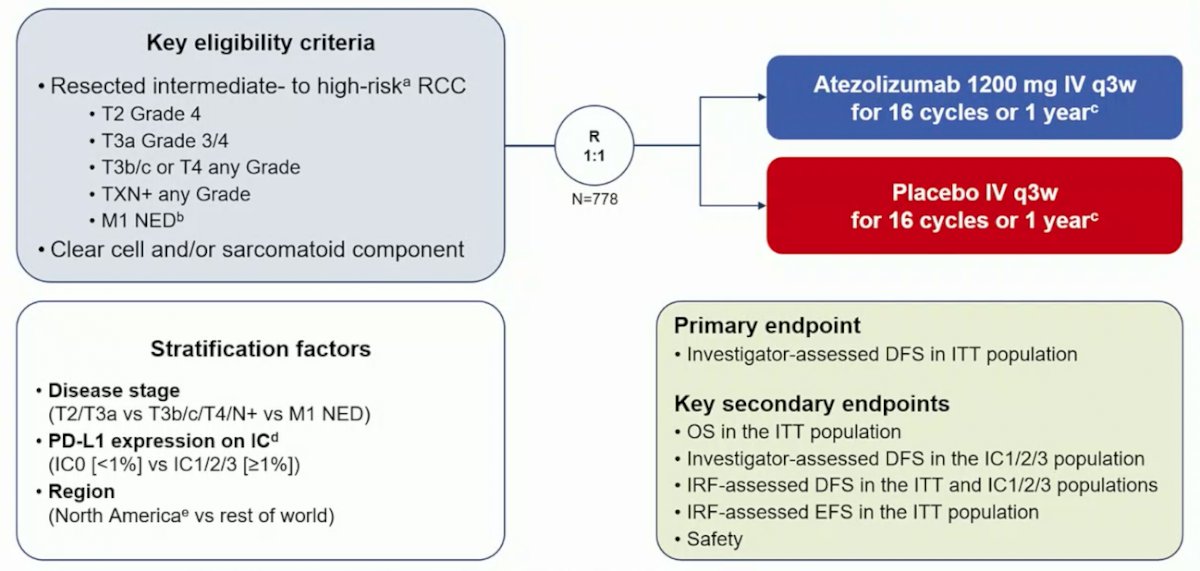
The primary endpoint was investigator-assessed disease-free survival (DFS). Secondary endpoints included OS and independent review facility (IRF)-assessed DFS in the ITT population, and investigator-assessed-DFS and IRF-DFS in patients with PD-L1 immune cell expression ≥1% (VENTANA SP142 IHC assay). After 38.6 months minimum follow-up, 332 investigator-assessed DFS events occurred, and these events resulted in 90% power to detect an improvement in DFS at a 0.05 significance level (2-sided) in 778 patients, assuming an HR of 0.70 and 5% loss to follow-up over 24 months. DFS median survival time was estimated using Kaplan-Meier methodology, with HRs and 95% CIs were calculated using stratified Cox proportional hazards model.
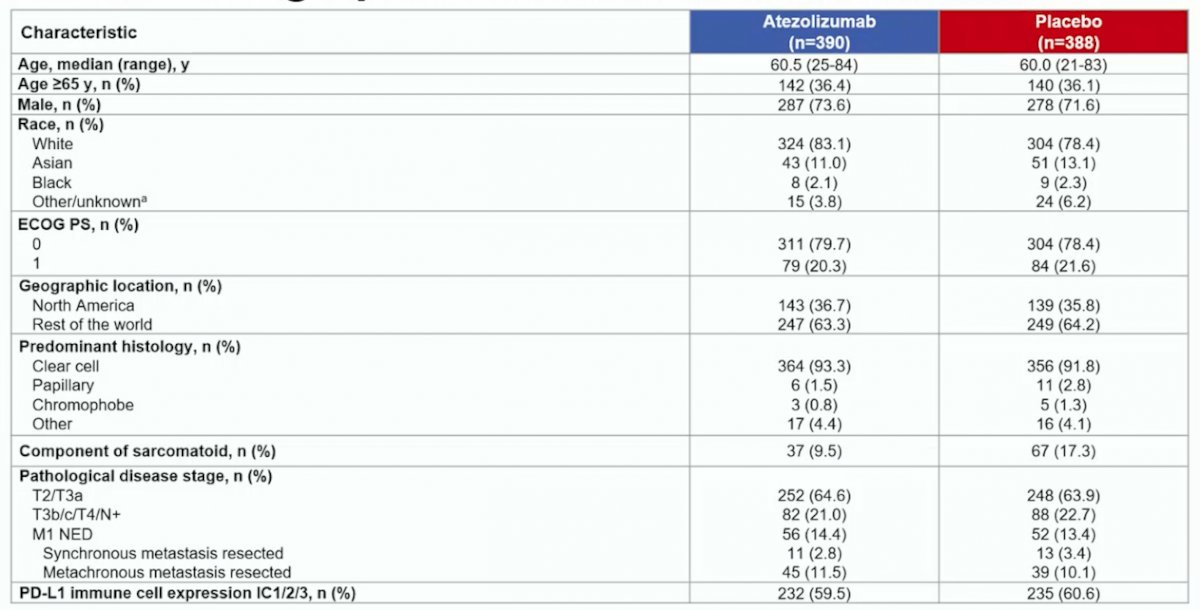
From January 3, 2017 to February 15, 2019, 778 patients were randomized to atezolizumab (n=390) or placebo (n=388). At primary analysis data cutoff (May 3, 2022), median follow-up was 44.7 months and minimum follow-up was 38.6 months. No patients remained on study treatment. Baseline characteristics were generally balanced between arms and shown as follows:
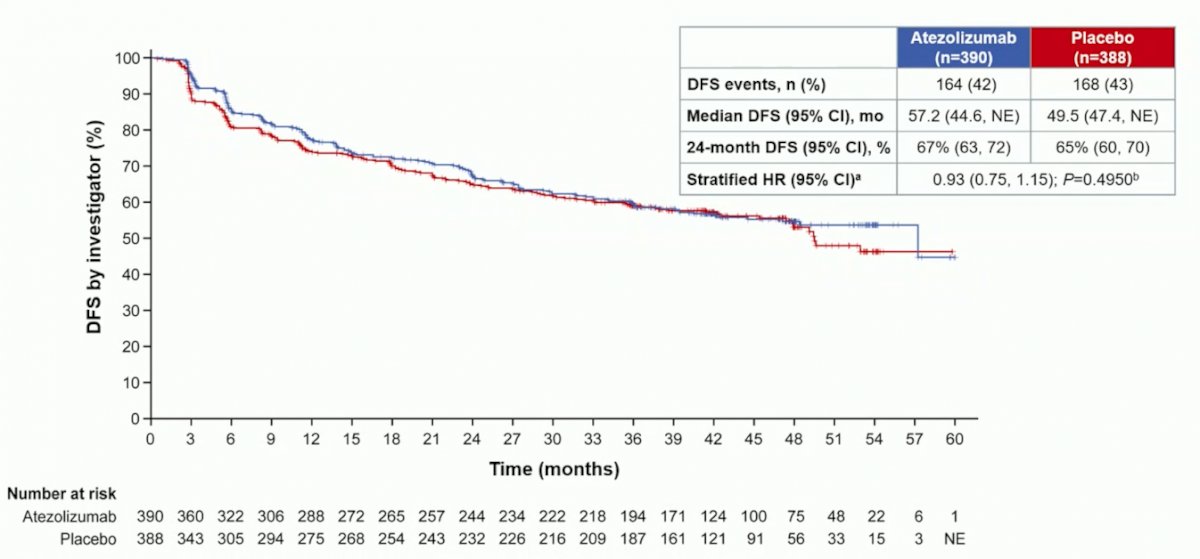
Median investigator-assessed-DFS was 57.2 months (95% CI 44.6, NE) for atezolizumab and 49.5 months (95% CI 47.4, NE) for placebo (HR 0.93, 95% CI 0.75, 1.15; p = 0.495):
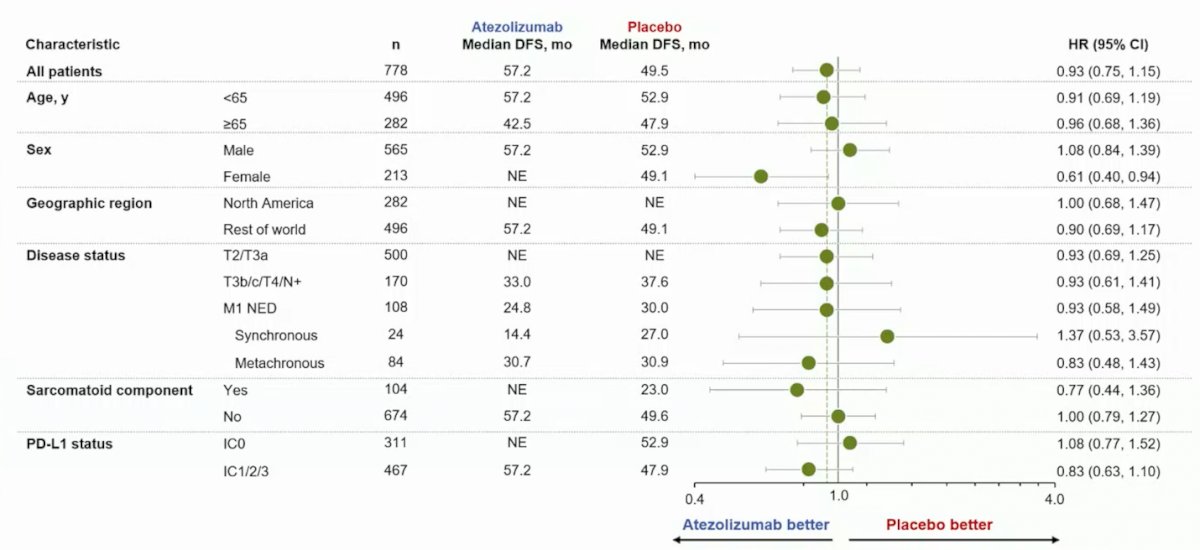
Looking at an investigator-assessed DFS subgroup analysis, there was minimal signal for benefit patients, albeit for females who may benefit from adjuvant atezolizumab (HR 0.61, 95% CI 0.40-0.94):
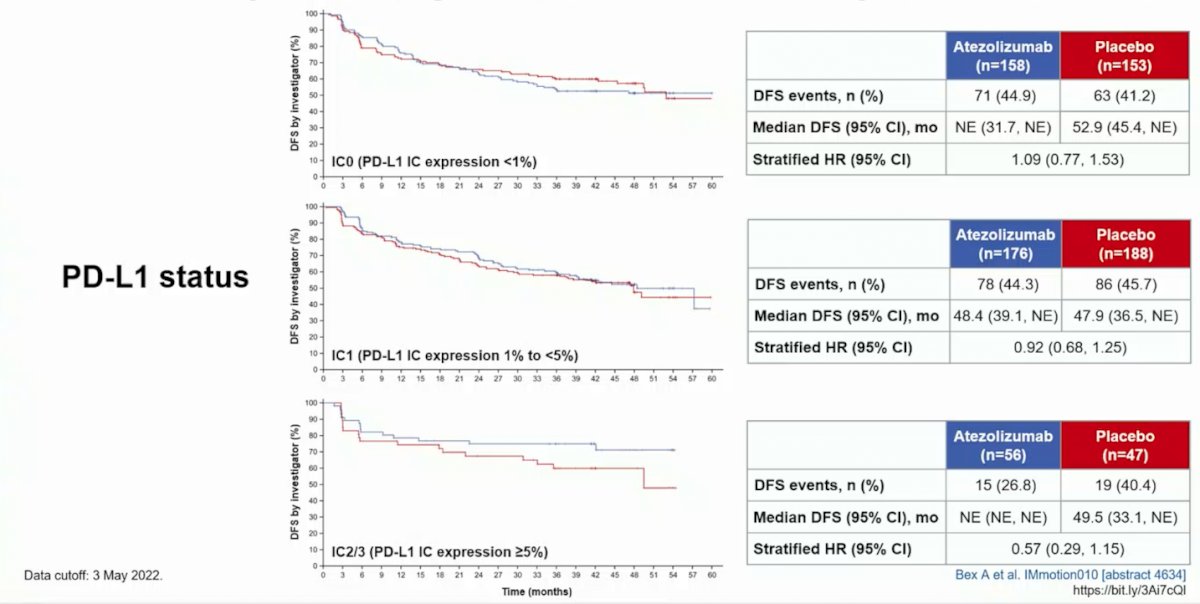
With regards to OS, data was immature, with the median OS not reached in either arm (stratified HR atezolizumab vs placebo HR 0.97, 95% CI 0.67-1.42). Exploratory analyses of investigator-assessed DFS by PD-L1 status suggests that there may be an efficacy signal among patients with IC2/3 (PD-L1 IC expression >= 5%):
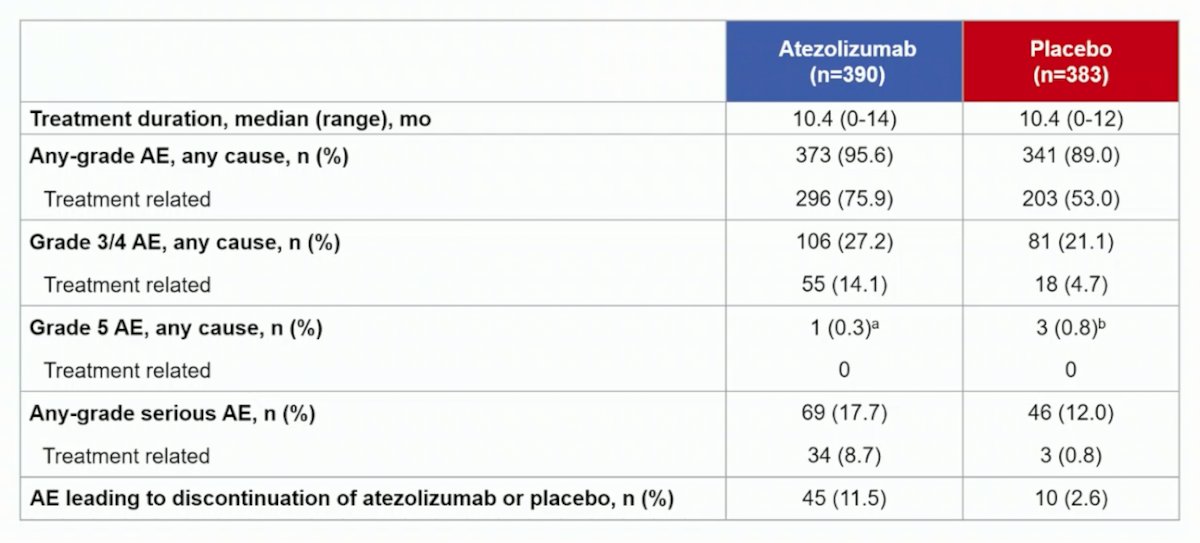
In the safety population, grade 3/4 adverse events occurred in 27.2% (106/390) and 21.1% (81/383) of patients receiving atezolizumab or placebo, respectively. Grade 5 adverse events occurred in <1% (1/390) and <1% (3/383), none related to treatment:
Dr. Bex concluded his presentation of IMmotion010 with the following take home messages:
- Atezolizumab as adjuvant therapy after resection for patients with RCC with increased risk of recurrence did not improve clinical outcomes vs placebo in the ITT population
- Atezolizumab was well tolerated, and safety results were consistent with the known safety profile of atezolizumab
- Subgroup analysis suggests further evaluation of sarcomatoid and high-expression PD-L1 populations is warranted
- Biomarker analyses are ongoing to identify patients who may benefit from adjuvant atezolizumab
- Further studies are needed to clarify the role of immunotherapy in the adjuvant setting for RCC
At the conclusion of Dr. Bex’s presentation, IMmotion010 was concurrently published in The Lancet.3
Presented by: Axel Bex, MD, PhD, Department of Urology, The Royal Free London NHS Foundation Trust, University College London Division of Surgery and Interventional Science, London, UK & The Netherlands Cancer Institute, Amsterdam, Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 European Society of Medical Oncology (ESMO) Annual Hybrid Meeting, Paris, FR, Fri, Sept 9 – Tues, Sept 13, 2022.
References:
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375(23):2246-2254.
- Choueiri TK, Tomczak P, Park SH, et al. Adjuvant Pembrolizumab after Nephrectomy in Renal-Cell Carcinoma. N Engl J Med. 2021 Aug 19;385(8):683-694.
- Pal SK, Uzzo R, Karam JA, et al. Adjuvant atezolizumab versus placebo for patients with renal cell carcinoma at increased risk of recurrence following resection (IMmotion010): A multicentre, randomized, double-blind, phase 3 trial. Lancet 10 Sept 2022 [Epub ahead of print].


