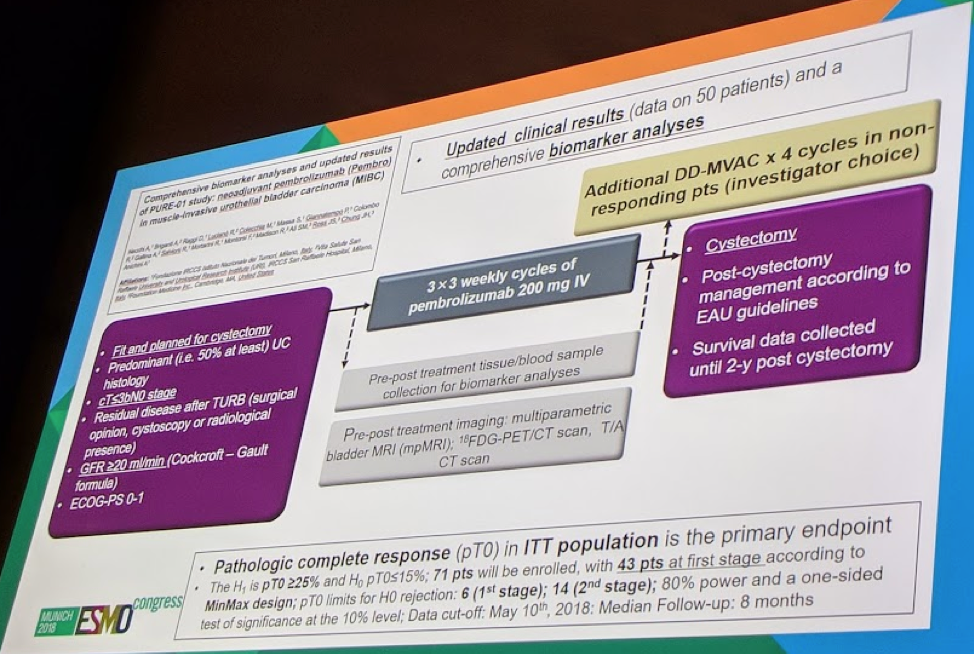
The planned enrollment is for 71 patients, all of whom will have cT ≤ 3b N0 M0 MIBC, regardless of cisplatin eligibility. Pembro will be given 200mg q3w x3 cycles. Pathologic complete response (pT0) in ITT population is the primary endpoint (EP). Biomarker analyses include: IHC PD-L1 combined positive score (CPS, Dako 22C3), hybrid-capture based comprehensive genomic profiling (CGP, FoundationONE), and expression of a 22-gene “T-cell inflamed” signature via quantitative PCR (qPCR).
The full study protocol is below:
The study population is slightly younger than expected – median age 66. However, male patients and smokers predominate, as expected. 92% of patients were cisplatin eligible.
As of the time of presentation, 65 pts have been enrolled and all underwent CGP from TURBT samples. 42% showed DDR genomic alterations (GA). Median CPS was 21%. CPS and qPCR showed a significant correlation (r = 0.71, p < 0.0001), whereas CPS did not correlate with tumor mutational burden (TMB) or DDR-GA (R=-0.16).
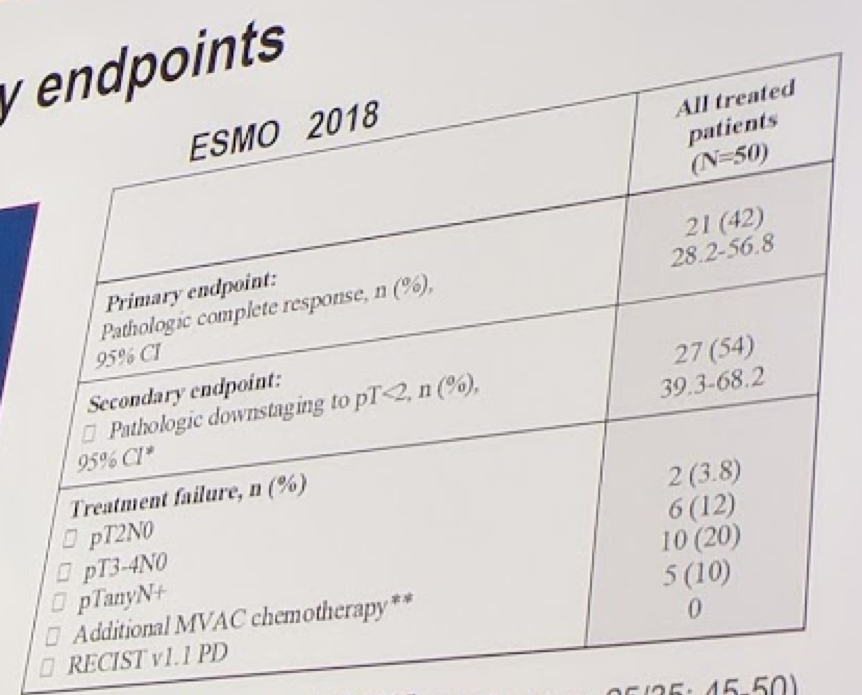
In terms of clinical outcomes, 50 pts are evaluable for the primary endpoint. With 21 (42%) pT0 responses, the study has already achieved its PE. The clinical outcomes are shown below:
RB1 and PBRM1 GA were significantly associated with pT0 (p = 0.014 and p = 0.007). pT0 responses were obtained in 10 (52.6%) pts with CPS≥21% and, most noteworthy, in 13 (61.9%) with DDR or RB1 GA. 8/8 pts (100%) with DDR/RB1 GA and CPS≥21% achieved pT0.
However, the DDR and RB1 gene association was weakened when accounting for tumor mutational burden. So they may just be a surrogate of TMB.
Using a CPS score cutoff of 10%, they found that patients who had CPS >= 10% had a 54% chance of pT0, while those <10% had a 13% chance of pT0.
The 22 gene T-cell inflamed signature also significantly discriminated pT0 from non-pT0 pts (p = 0.0032).
17 pts had matched pre-post Pembro tumor samples analyzed, showing a mean of 51.9% shared GA. Concordant increases in gene expression by qPCR, observed in post- vs pre-Pembro lesions, from at least 5/7 non responding patients, were consistent with promotion of adaptive immunity (IFN-g, CXCL9, CXCR6, CD27, GZMB), being counteracted by strong adaptive resistance mechanisms (CD274, PDCD1, CD276, PDCD1LG2, IDO1).
This study is exciting because it would appear that pembro has already exceeded the pT0 responses required. The authors suggest that using many of these genomic observations of predictors of response to pembro may help identify patients who might deserve a bladder-sparing approach.
Ignacio Duran, MD, PhD, had the following to add.
Neoadjuvant chemotherapy for muscle-invasive bladder cancer is an established treatment paradigm supported by Level 1 evidence. Historically utilizing an MVAC regimen, there was subsequently a shift to Gem-Cis and, more recently dose-dense MVAC (ddMVAC). Indeed, these studies have repeatedly demonstrated good tolerability with the dose-dense regimens. Meta-analyses put the increased survival benefit at 5-7%, if all comers are treated. However, the greatest benefit is in the population of patients with complete response (pT0) at the time of cystectomy; however, even patients with residual non-muscle-invasive (<pT2 disease) do better than patients not receiving chemotherapy. However, patients with no response (pT2-T4 disease) do similar to patients who never received chemotherapy. Hence, the pressure is on to select out the patients who may not respond – and see if alternative options exist.
Yet, alternative options (such as ICI’s) must be able to have a better pCR (pT0) rate than NAC (25-30%). They should also not delay surgery. And their toxicity profile should be manageable.
From these early results, it appears that pembro monotherapy in the neoadjuvant setting is able to match, even exceed, the pT0 rate associated with NAC, albeit in low numbers.
- PD-L1 >= 10% appears to be useful in selecting out patients most likely to benefit
- They don’t appear to delay surgery
While this is certainly exciting – it is not yet practice-changing. NAC is recommended based on Level 1 evidence. That data does not yet exist for ICI’s. Randomized trials are required to validate these findings.
Presented by: Andrea Necchi, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Invited Discussant: Ignacio Duran, MD, PhD, Hospital Univ. Virgen del Rocío, Instituto de Biomedicina de Sevilla, Seville, Spain
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @TjuUrology) at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany


