(UroToday.com) The treatment for metastatic hormone-sensitive prostate cancer plenary session at the European Association of Urology (EAU) 2021 annual meeting included a presentation by Dr. Piet Ost discussing radiation therapy for local treatment to the primary +/- to the lymph nodes.
Dr. Ost started by emphasizing that based on data from arm H of the STAMPEDE trial,1 radiotherapy in patients with low-volume metastatic disease is the standard of care. In this trial, radiotherapy improved failure-free survival (HR 0.76, 95% CI 0.68-0.84) but not OS (HR 0.92, 95% CI 0.80-1.06). In a prespecified subgroup analysis, patients receiving radiotherapy to the prostate among patients with low metastatic burden, there was a significant improvement in OS (HR 0.68, 95% CI 0.52-0.90).
Dr. Ost also highlighted data published earlier this year assessing the association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer.2 This was an exploratory analysis of treatment outcomes based on metastatic site and extent within the STAMPEDE trial's metastasis M1 radiotherapy comparison. A total of 1,939 men were included, of which 1,732 (89%) had bone metastases. Bone metastasis counts were associated with overall survival and failure-free survival benefit from prostate radiotherapy. Survival benefit decreased continuously as the number of bone metastases increased, with benefit most pronounced up to 3 bone metastases. Further analysis based on subgroups showed that the magnitude of benefit from the addition of prostate radiotherapy was greater in patients with low metastatic burden with only nonregional lymph nodes (M1a) or 3 or fewer bone metastases without visceral metastasis (HR for OS 0.62, 95% CI 0.46-0.83; HR for FFS 0.57, 95% CI, 0.47-0.70) than among patients with 4 or more bone metastases or any visceral/other metastasis (HR for OS 1.08, 95% CI, 0.91-1.28; interaction p = 0.003; HR for FFS 0.87, 95% CI, 0.76-0.99; interaction p = 0.002):
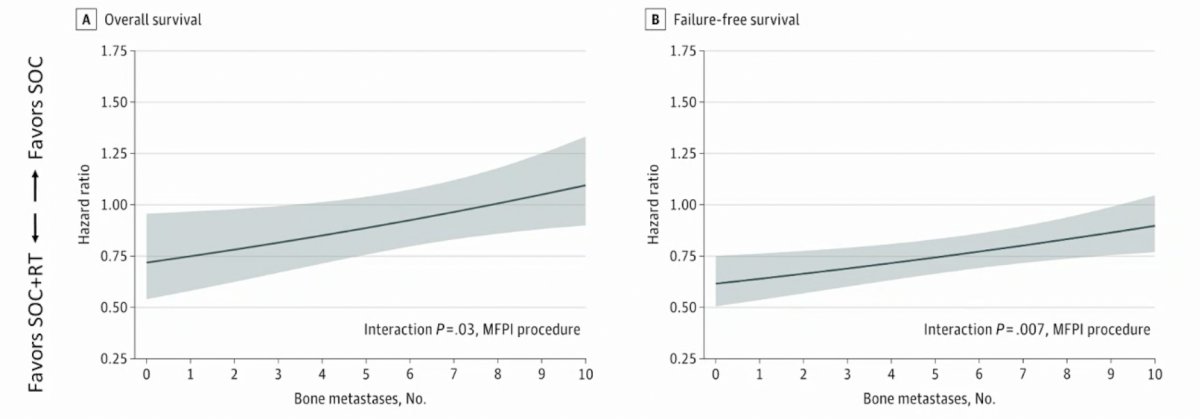
As such, this has led to a new definition of low burden of metastatic disease, defined as patients with or without non-regional lymph node disease regardless of axial or extra-axial location and without and visceral/other metastasis. Essentially, high burden of disease is everyone else that does not fit this definition. For low burden disease, the question remains as to whether to add radiotherapy or systemic therapy. For docetaxel, STAMPEDE uses ADT plus docetaxel first, followed by radiotherapy, whereas for the ARPIs there is no data (although this is being evaluated in the PEACE 1 trial). In cases where you want to give radiotherapy, Dr. Ost recommends not postponing treatment.
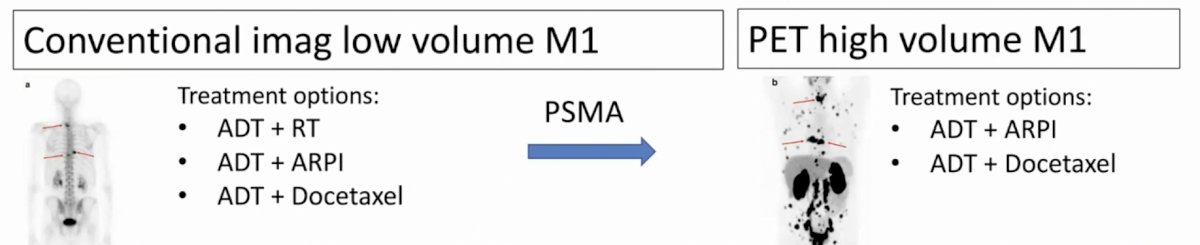
With regards to PSMA PET/CT, next-generation imaging will change management in patients. For a patient that is conventional imaging M0, treatment options include radical prostatectomy or radiotherapy +/- ADT, whereas after PSMA PET/CT they be upstaged to PET low volume M1 and treatment options now include ADT + radiotherapy, ADT + ARPIs, or ADT + docetaxel:
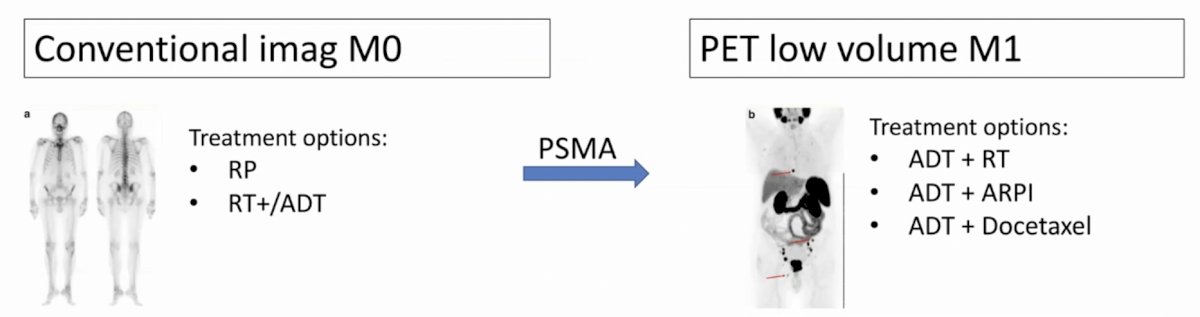
Furthermore, for patients that are conventional imaging low volume M1, treatment options include ADT + radiotherapy, ADT + ARPI or ADT + docetaxel and after PSMA PET/CT in situations where a patient is upstaged to PET high volume M1 disease, options now include ADT + ARPI or ADT + docetaxel:
Dr. Ost concluded his presentation with the following summary statements:
- Prostate radiotherapy + ADT (+/- 6 cycles of docetaxel) improved overall survival and failure-free survival in patients with only lymph node or <4 bone metastases (+/- lymph node) regardless of location
- However, there are several open issues such as:
- How do we combine with systemic therapy?
- What is the optimal target for radiotherapy? Primary only, primary plus nodes, primary plus nodes plus metastases?
- What about PSMA PET/CT?
Presented by: Piet Ost, MD, PhD, University of Ghent, Ghent, Belgium
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Ali A, Hoyle A, Haran AM, et al. Association of Bone Metastatic Burden with Survival Benefit from Prostate Radiotherapy in Patients with Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Onc 2021 Apr 1;7(4):555-563.