He first focused on the molecular subtyping work that has been driving the translational work in MIBC. There are 4 major molecular classifications based on work done by 4 different groups: MDACC, Lund, UNC and TGCA. There are two major categories: basal/luminal. While each of the molecular classifications vary in terms of naming and terminology, they generally adhere to this major subdivision.
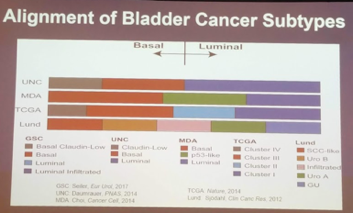
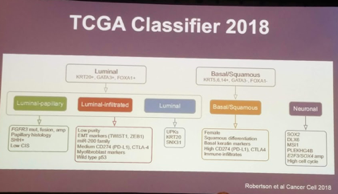
It should be noted that just this year, Robertson et al. (Cancer Cell 2018) described a 5th smaller subtype called neuronal/neuroendocrine, using TCGA data. On retrospective evaluation, patients with the genomic profile appeared to do worse with all treatment modalities (chemotherapy, surgery) – with the sole exception of chemoradiation!
However, these classification systems are based on using large datasets, unsupervised clustering and grouping. This is not practical for a patient sitting in the clinical.
At this time, neoadjuvant chemotherapy (NAC) is a standard of care based on Level 1 evidence prior to radical cystectomy for MIBC. While there is a lot of data to support the use of NAC, there are two major gaps in its widespread utilization:
• Only 40% of patients have major response to chemotherapy
• NAC is not widely used in most parts of the world
The best response to these gaps in care is better risk stratification and patient selection for chemotherapy response, with the use of biomarkers.
Three different molecular markers to discuss:
1. Molecular subtypes
2. COXEN model
3. Genomic alterations
Molecular subtypes – described above, but have been shown to be associated with clinical outcomes and response to therapy.
Subtype is associated with response to chemotherapy
o Basal tumor respond very well
o P53-like tumors respond poorly
Dr. Black worked with Genome Dx to generate a genomic test that could classify a patient sitting in front of you in clinic into 1 of 4 molecular classes
o Using discovery and validation set, proved that basal molecular subtypes are the best responders to NAC cisplatin
o Luminal tumors do well regardless of NAC – so maybe they don’t need chemotherapy
COXEN Model “Coexpression Extrapolation”
• Being developed based on 60 cell lines and their drug response
• Test a patient’s gene expression against the pool of cell line data to determine the best “match”
• Shown an accuracy ~80%
• Currently in a prospective study with SWOG
Genomic Alterations
• Individual Gene alterations, particularly DNA repair genes
• Will likely gain more recognition as predictors of response
• I.e. ERCC2 gene alterations were found only in patients with chemotherapy response – validated in a second cohort in FCCC
⁃ Therefore those patients with ERCC2 mutations treated with chemotherapy do well
⁃ Mutations in ATM, Rb1, FANC – 3 gene panel
• Patients with mutation in at least one of these genes had a high rate of response to chemotherapy
• ERBB2 (Her2)
• Predictive of chemotherapy response, but not yet validated
He did focus a little bit on combining some of these biomarkers – specifically by including patients with basal subtype and patients with DNA damage repair gene mutations, approximately 50% of patients would be considered potential NAC responders. This may help select out patients who wouldn’t respond to NAC and avoid unnecessary treatment.
⁃ In early, retrospective analyses of a TCGA non-NAC cohort and a separate institutional NAC therapy cohort, there did appear to be a prognostic value to having either basal subtype of DDR gene mutations – better response and survival seen with NAC administration.
However, all of these markers have yet to be validated in prospective clinical trials. When they are validated, they should prioritize marker positive patients for NAC and marker-negative patients for immediate cystectomy.
He lastly touched on patients treated with NAC – what is their genomic profile like? Dr. Black’s group has begun to address this already by profiling patients’ TURBT tissue (pre-NAC) and cystectomy specimens (post-NAC). Unsupervised consensus clustering yielded four distinct consensus clusters (CC) or subtypes – however, they did not match up perfectly with the original molecular subtypes. Two CC’s expressed genes known from previously described molecular subtypes of chemotherapy naïve BC: CC1 and 2 expressed genes consistent with a basal-like (KRT5/6, KRT14) and a luminal-like (GATA3, PPARG) phenotype and were called, CC1 basal and CC2 luminal, respectively. CC3 expressed a strong T-cell signature, markers for T-cell receptor signaling, chemokines and checkpoint molecules (CTLA4, CD80) and was therefore called CC3 immune. CC4 was associated with wound healing/scarring (MYH11, CNN1). This ‘scar-like’ character of CC4 was highly consistent with the scar samples (n=21) – and these patients did very well, likely representing a genomic marker of strong NAC response.
The future is bright for bladder cancer therapeutics, and hopefully, many of these advances will lead to clinical impact soon.
Presented by: Peter Black, MD, University of British Columbia, Vancouver, BC
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, Twitter: @tchandra_uromd at the 73rd Canadian Urological Association Annual Meeting - June 23 - 26, 2018 - Halifax, Nova Scotia


