Case #1 – Treating CIS: BCG and Beyond
This was a 79-year-old man with an 8-month history of irritative lower urinary tract symptoms and microscopic hematuria (50 RBCs/HPF). His past medical history was consistent with a DVT with PE, laryngeal cancer s/p resection, hearing loss, and diabetes mellitus. Of note, this patient had a 30 pack-year smoking history and a creatinine of 1.5. A CT urogram was negative for upper tract disease, in-office cystoscopy showed two 2 cm papillary lesions, and cytology was suspicious for high-grade urothelial carcinoma.
Joshua Meeks, MD, Ph.D., notes that urine cytology in these situations is somewhat debatable, but that given this patient’s history he would obtain cytology. This may dictate whether an intraoperative dose of intravesical chemotherapy is warranted (after TURBT), and he notes that many of the non-muscle invasive bladder cancer trials do require cytology for inclusion criteria. Sima Porten, MD, MPH, notes that for these patients she likes to use blue-light cystoscopy in order to detect hidden CIS. This patient had HG Ta and CIS at the time of his TURBT.
Currently, in the US we have had intermittent BCG shortage, so there is debate as to what to use in times of BCG shortages. Dr. Porten notes that there are several things we can do:
- Address modifiable risk factors for recurrence, such as smoking cessation
- Perform a high-quality TURBT
- Ration BCG, focusing on those that will benefit most, including those with CIS or other high-risk features, as well as reduce the concentration dose of BCG (up to 1/3) and the dose number for each cycle (for example, 5 doses instead of 6 for induction or 2 doses instead of 3 for maintenance). Also, one may forego maintenance and we should not use BCG for low-risk patients
- Proceed to cystectomy (especially for those with high-risk disease, HG T1 +/- CIS, variant histology)
- Use intravesical chemotherapy (for example gemcitabine, mitomycin C, combination chemotherapy)
- Enroll in clinical trials
Dr. Chang notes a word of caution in that if we don’t give enough BCG, this can actually be harmful. Presented earlier this year at GU ASCO, data from the NIMBUS trial suggests that reduced frequency of BCG during induction and maintenance was associated with worse recurrent-free survival compared to the standard frequency of BCG (57% worse outcomes). Intravesical doublet chemotherapy is an attractive option for these patients, as Dr. Porten notes that gemcitabine plus docetaxel was associated with a 50% 1-year recurrence-free survival rate (81% with maintenance) and 20% 2-year recurrence-free survival rate (59% with maintenance).1
This patient ultimately underwent six weeks of induction BCG, followed by a negative white light surveillance cystoscopy and negative cytology. He then underwent three weeks of maintenance BCG, followed by a negative blue light surveillance cystoscopy and negative cytology. Subsequently, he underwent three weeks of additional maintenance but had severe lower urinary tract symptoms towards the end of this course that necessitated a dose reduction for the last dose. Dr. Meeks notes that oftentimes he will use anticholinergic medications to keep from having to limit BCG dose, but this often does not help the patient’s symptoms. This patient subsequently was found to have BCG unresponsive CIS. Dr. Porten highlights the treatment options for BCG unresponsive CIS as follows: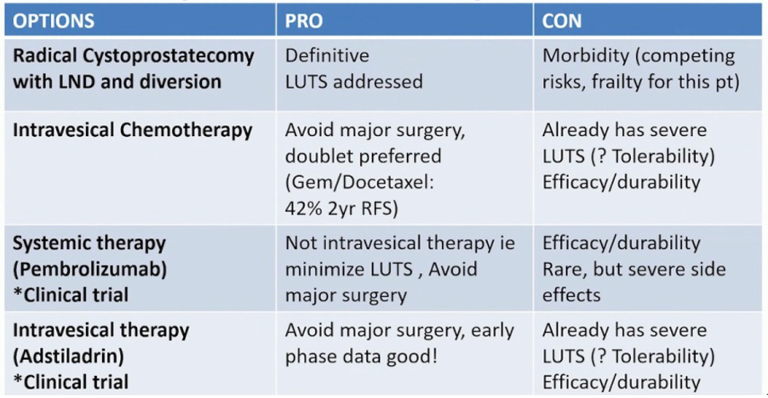
The 2016 AUA Guideline Statement supports radical cystectomy, particularly for highest risk patients (for example HGT1, BCG unresponsive). Patients with T1 recurrence after BCG who undergo radical cystectomy have better DSS (69%) than those who are treated with resection plus intravesical therapy (52%). However, Dr. Chang notes that despite advances in perioperative management and surgical technique, there are still complications, morbidity, and mortality associated with radical cystectomy. Furthermore, patients may refuse surgery or unfit to undergo radical cystectomy. Bladder sparing options include the recently FDA approved pembrolizumab for recurrent CIS after BCG (KEYNOTE 057), first presented at the 2019 ASCO GU meeting and discussed today by Dr. Plimack. Among patients that respond to pembrolizumab, the median duration of response is 16.2 months (range 0-30.4), with complete response rates at 3 months of 40.2% and no progression to T2 disease. She cautions that it will be important to see the follow-up data at 3 and 5 years, to ensure that we are truly curing these patients of their disease.
Additional trials are ongoing in this disease space, including the single-arm, open-label trial evaluating Nadofaragene firadenovec (Adstiladrin®)) in high-grade, BCG-unresponsive non-muscle invasive bladder cancer. Initial data showed that 53.4% of patients enrolled in this trial (n=103) had complete response rates at 3 months. Furthermore, at the 12-month follow-up mark, 45.5% of those with CIS +/- HG Ta/T1 achieving an initial complete response had durable complete responses.
There are several key points for CIS disease as highlighted by Dr. Porten:
- Although BCG is the recommended therapy, its lack of availability reinforces the need for alternatives
- In the BCG-naïve patient, this includes intravesical chemotherapy
- In the BCG-unresponsive patients, this includes intravesical chemotherapy, systemic pembrolizumab, as well as other possible future intravesical agents
- Enhanced cystoscopy and careful TURBT are essential
Case #2 – Challenges of T1 Bladder Cancer: Interpreting Heterogeneity
This was an 82-year-old man with gross hematuria and left flank pain, with a medical history of diabetes, hypertension, and coronary artery disease. He had a 50-pack year smoking history and a creatinine of 1.5. His CT scan showed a 3.6 cm bladder mass, mild left hydronephrosis, no lymphadenopathy. At the time of his TURBT, tumor was noted to be obscuring his left ureteral orifice, which was not identifiable. His pathology from the TURBT showed T1 high-grade disease with detrusor muscle present but not involved and confirmed by re-review with a secondary genitourinary pathologist (no lymphovascular invasion and no variant histology). A percutaneous nephrostomy tube was subsequently placed.
Dr. Meeks notes the importance of a re-TURBT in these patients. In this patient, repeat TURBT demonstrated HG T1 with residual tumor at the left ureteral orifice; he was also able to exchange his percutaneous nephrostomy tube for a double J stent, with resolution of the hydronephrosis. Another TURBT four weeks later showed LG Ta. Dr. Chen notes that for HG T1 disease patients, they do not typically offer radiation for these patients. Dr. Meeks stated that this patient was subsequently enrolled in the S1602 “PRIME” trial, which is highlighted as follows: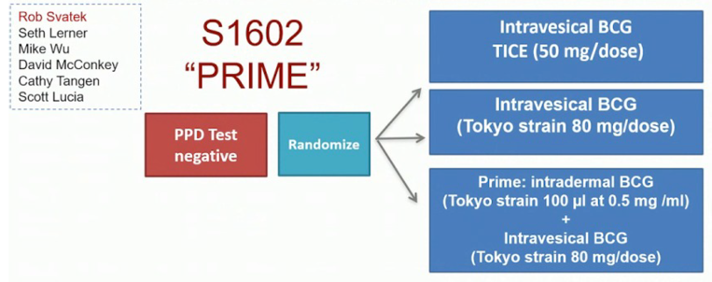
This patient was then noted to have a recurrent blue-light positive bladder tumor. His treatment summary to this point is as follows:
- TURBT: T1HG, muscle present and negative
- TURBT: T1HG, muscle present and negative
- TURBT: TaLG, muscle present and negative
- BCG x 6 weeks, cystoscopy negative
- BCG x 3 weeks, mass found at cystoscopy
- TURBT: T1HG, muscle present, CT without lymphadenopathy and stent exchanged
Ronald Chen, MD, MPH, notes that because the patient is still not muscle-invasive the modality of choice would be radical cystectomy, however, he stated that patients that are unfit for cystectomy may be treated with chemoradiation as per the NCCN guidelines. Dr. Plimack notes that there is no solid role for neoadjuvant chemotherapy for this patient, as this is typically reserved for patients with muscle-invasive disease. The patient subsequently underwent a radical cystectomy, with pathology high-grade pT2aN0 (0/14) with negative margins. Dr. Meeks feels that there is likely different biology for T1 tumors compared to Ta tumors, given that there is a degree of invasion with T1 disease (heterogeneity of level of invasion). Additionally, assessing the true nature of T1 disease may be challenging in that we may understage the patient and/or there are issues with cautery artifact. He notes that it is easy to “miss the boat” on these patients and that it is important to follow the AUA guidelines for HG T1 patients: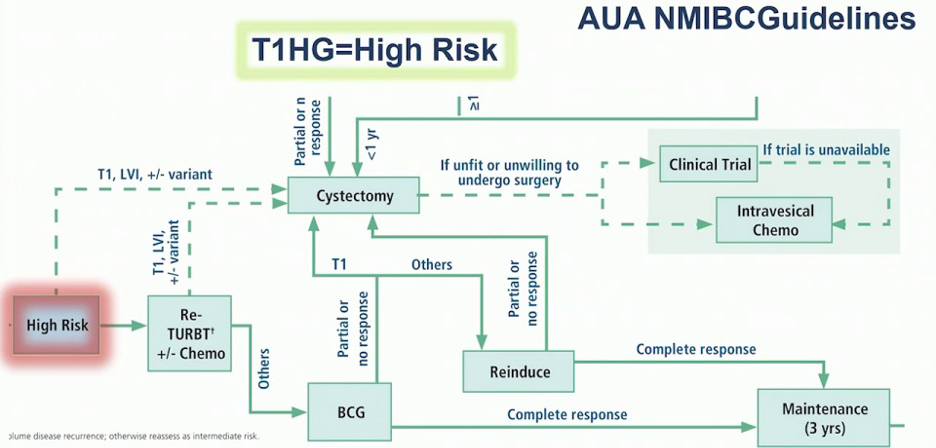
Dr. Meeks provided several key points for T1 HG bladder cancer:
- Repeat TURBT is still the standard of care
- We should be aware of variant pathology and those with lymphovascular invasion
- Certain patients should be considered for early cystectomy
- BCG with maintenance should be considered for most patients
- We should beware of early recurrences and consider cystectomy, new agents, and clinical trials
Case #3 – Bladder Preservation for Muscle-invasive bladder cancer: Survival Comparisons with Cystectomy
This was a 56-year old man with painless gross hematuria and a history of hypertension and nephrolithiasis. His eGFR was >60 and his CT scan showed no masses, hydronephrosis, or lymphadenopathy. At TURBT he was found to have a 2-cm bladder floor mass and was noted to have cT2 disease.
Dr. Chen notes that there are several components to trimodality therapy, including a maximum safe TURBT, radiation therapy, and concurrent chemotherapy. Options for chemotherapy include cisplatin-based regimens, 5-FU/mitomycin C, or gemcitabine. There are currently multiple prospective phase II and III trials underway, and previous data suggest that among long-term survivors, bladder preservation is possible in up to 70% of patients. Although there is no head to head trials comparing radical cystectomy and trimodal therapy, a summary of prospective data suggests a ~50% 5-year survival for both therapy options: 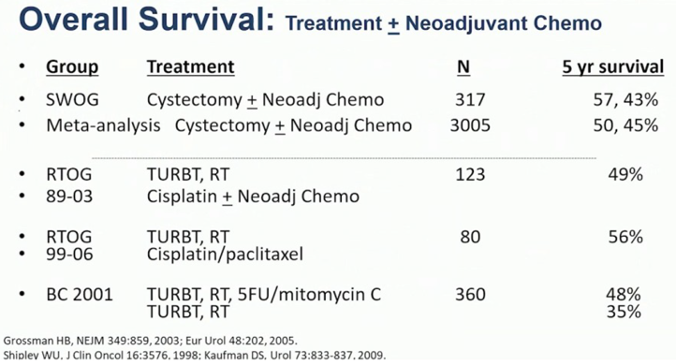
Aggregative data from the RTOG experience (89-03, 95-06, 97-06, and 99-06) suggests that over a median follow-up of 5.4 years, the grade 3 GI toxicity rate is only 1.9%, grade 3 GU toxicity rate is 5.7%, and there were no late grade 4-5 toxicities.2
According to Dr. Chen, the following criteria are paramount for high success rates for multimodality therapy:
- Solitary T2 or early T3 tumors < 6cm
- No tumor-associated hydronephrosis
- Tumors allowing a visibly complete TURBT
- Invasive tumors not associated with extensive carcinoma in situ
- Ability to tolerate concurrent chemotherapy
Dr. Chen highlights the importance of clinical trials in this disease space, noting the SWOG/NRG 1806 trial: 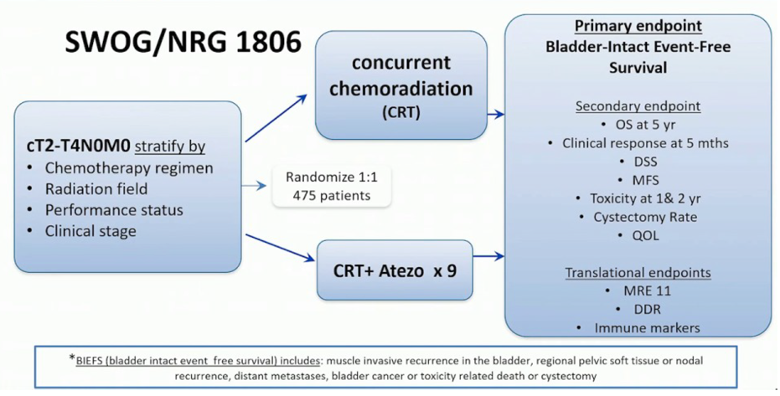
Bladder preservation take-home messages were provided by Dr. Chen as follows:
- Trimodality therapy with curative intent is effective
- Radiation alone for patients who cannot tolerate concurrent chemotherapy can offer some benefit
- Patients who cannot tolerate a cystectomy or who chose not to undergo a cystectomy should be offered multidisciplinary counseling
- Disease and patient factors can influence outcomes
Elizabeth Plimack, MD, MS, concluded this case-based discussion with several highlights from the advanced bladder cancer disease space:
- In the last five years, we have seen five immune checkpoint inhibitors approved
- In the last year alone we have had two targeted therapies approved: enfortumab vedotin and erdafitinib
- Clinical trials are continuing to enroll in the muscle-invasive (neoadjuvant), adjuvant, first-line metastatic, and subsequent lines of therapy disease spaces
Moderator: Sam Chang, MD, Vanderbilt University, Nashville, TN
Panelist: Elizabeth Plimack, MD, MS, Fox Chase Cancer Center, Philadelphia, PA
Panelist: Sima Porten, MD, MPH, UCSF, San Francisco, CA
Panelist: Joshua Meeks, MD, Ph.D., Northwestern University, Chicago, IL
Panelist: Ronald Chen, MD, MPH, University of North Carolina, Chapel Hill, NC
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 American Urological Association (AUA) Annual Meeting, Virtual Experience #AUA20, June 27- 28, 2020
- Milbar N, Kates M, Chappidi MR, et al. Oncological Outcomes of Sequential Intravesical Gemcitabine and Docetaxel in Patients with Non-Muscle Invasive Bladder Cancer. Bladder Cancer 2017 Oct 27;3(4):293-303.
- Efstathiou JA, Bae K, Shipley WU, et al. Late pelvic toxicity after bladder-sparing therapy in patients with invasive bladder cancer: RTOG 89-03, 95-06, 97-06, 99-06. J Clin Oncol 2009 Sep 1;27(25):4055-4061.
AUA Guidelines Case-Based Panel Discussion: Bladder Cancer (Non-muscle and Muscle Invasive) - Joshua Meeks and Sima Porten


