According to Scott A. MacDiarmid, MD, nocturia has a high prevalence in the adult population, but it’s undiagnosed and undertreated (Figure 1). It is known to negatively affect people’s quality of life because it is linked to sleep deprivation, reduced work productivity, increased mortality and morbidity such as increased risk of cardiovascular events and increased risk of falls and fractures. Thomas Roth, PhD reported that individuals with nocturia sleep an average of 2-3 hours before being awoken due to their need to void. However, a person needs a minimum of 4 hours of uninterrupted sleep to be considered restorative. A recent study showed that more than 40% of people who wake up at night to void have difficulty going back to sleep and demonstrate an increased daytime sleepiness.

Figure 1.
Jennifer Miles-Thomas, MD reported that nature of nocturia depends on a variety of factors, which include bladder storage issues, polyuria, sleep disturbances, depression, anxiety, lifestyle choices (timing and quantity of fluid intake) and certain medications. Nocturnal polyuria (NP) is one of the most prevalent conditions diagnosed by health professionals, but current nocturia treatment options (behavioral modifications, OAB and BPH medications, and antidiuretics) are insufficient in improving NP symptoms.
NP is caused by the increased production of urine in kidneys at night. Noctiva is a first antidiuretic that increases water reabsorption. Medication is produced in a form of nasal spray, which contains a microdose of desmopressin (0.83 and 1.66 mcg of desmopressin acetate). High bioavailability of desmopressin allows for low dosing, which affects urine production for about 4-6 hours.
Two 12-week, Randomized, double-blind, placebo-controlled studies were conducted in order to evaluate drug’s safety and effectiveness. Study participants included men and women with an average age of 67, two or more nocturia episodes at night for at least 6 months, and at least 13 baseline nocturia episodes over 6 nights documented in the voiding diary. All eligible subjects received a dose of placebo for 2 weeks. Then they were randomized to one of 3 groups: 1.66 mcg of Noctiva, 0.83 mcg of Noctiva, or placebo. Primary endpoints included a change in mean number of nocturia episodes per night and a number of subjects with at least 50% reduction in mean nocturnal episodes in the course of 12 weeks. Secondary endpoints included a number of nights without voids and percentage of nights with 1 nocturic episode.
Data analysis revealed that dose of 1.66 mcg showed statistical significance in terms of highest percentage of patients (48%) who exhibited more than 50% reduction of nocturic episodes, decreased number of times awoken by need to void at night, and increased number of nights with one or less nocturic episode over the course of the study (Figure 2). Moreover, individuals who took Noctiva were more likely to stay in bed longer before waking up to void thus improving the overall quality of life. Although dose of 0.83 mcg was proven to be effective in one out of two trials, it still showed improvement in subjects’ symptoms. Placebo group participants also reported a decrease in nocturic episodes (27.5%). This can be explained by patients’ awareness of lifestyle modifications.
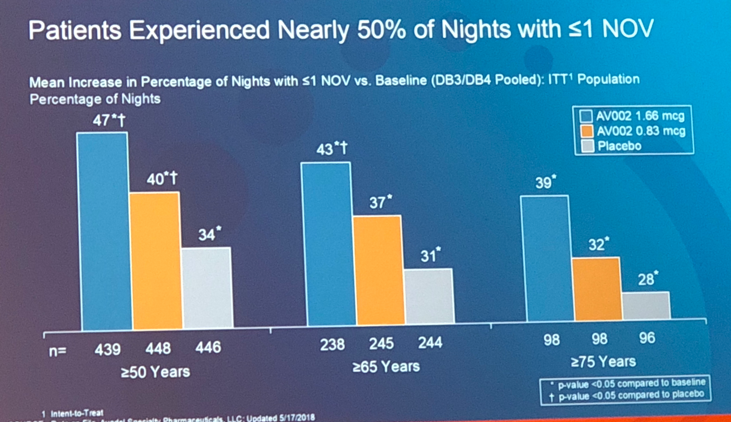
Separate long-term, open-label studies were performed to evaluate long-lasting effects of medication. Data showed that subjects who took 0.83 mcg of Noctiva experienced a decrease of about 2 voiding episodes per night over 40 weeks. Treatment with 1.66 mcg of Noctiva over 126 weeks caused a similar reduction of 2 nocturic episodes per night.
The Impact of Nighttime Urination Questionnaire (INUQ) was an important development covered in the presentation. It was created and validated in a separate study to analysis effects of nocturia on daily activities and nighttime behaviors. The INUQ consists of 10 items that cover questions on concentration, tiredness, ability to complete daily activities, mood, drowsiness, anxiety associated with nocturia, sleep disturbances, and disease bother. Overall score ranges 0 to 100, with 0 indicating no impact and 100 being greatest impairment. This tool was used in one of the Noctiva trials to assess the impact of nocturia at baseline and after study’s completion. Dose of 1.66 mcg showed the highest decrease in the score.
There were several adverse events reported in individuals who used Noctiva. Severe hyponatremia (serum sodium ≤125 mmol/L) was diagnosed in 5 out of 695 subjects (Figure 3). These 5 individuals on 1.66 cg of medication had following characteristics: all were 65 years of age or older; 4 men and 1 woman, 4 were talking concomitant glucocorticoids. Other significant adverse events included nasal discomfort, nasopharingitis, and hypertension.
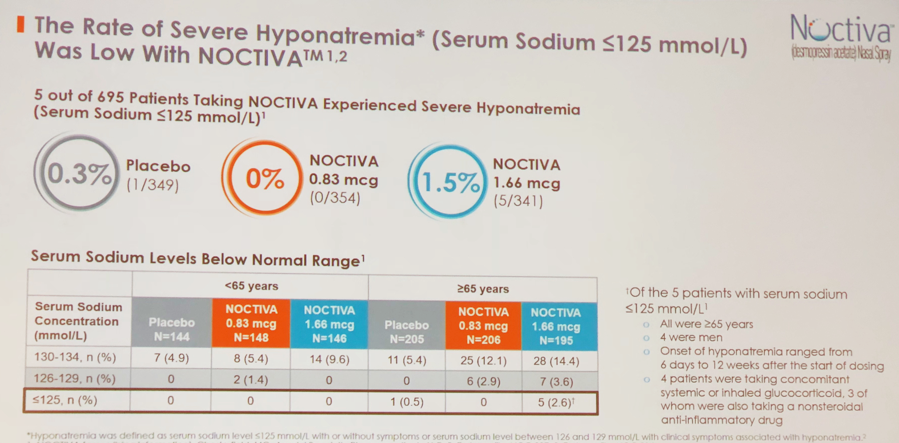
Figure 3.
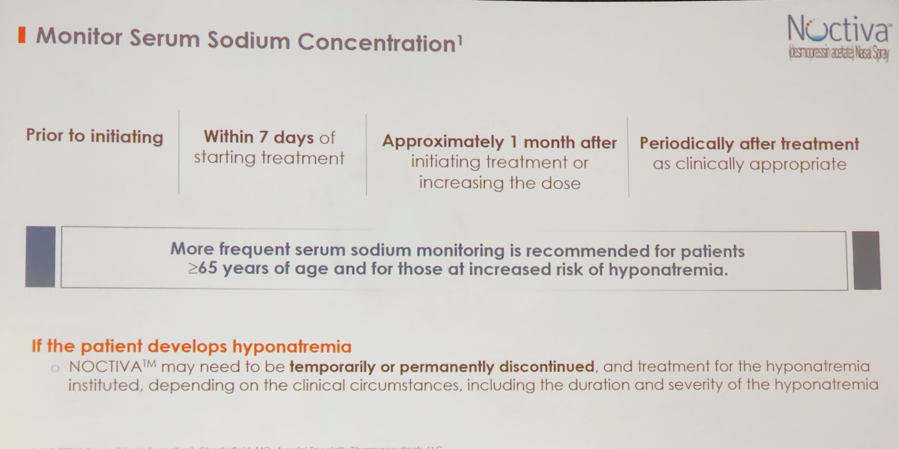
Diane Newman, DNP, reviewed current recommendations of Noctiva administration. Noctiva is available in 2 doses: 1.66 mcg for patients less than 65 years of age who don’t have an increased risk of hyponatremia and 0.83 mcg for people ≥65 years and younger individuals who are likely to develop hyponatremia. It is recommended to periodically evaluate serum sodium concentration after starting treatment, dose increase, and specifically in people at risk for hyponatremia (Figure 4).
Finally, a case study was reviewed to illustrate how Noctiva can be incorporated into the clinical practice as an only FDA-approved medication for the treatment of nocturia in adults with nocturnal polyuria.
Presented by: Scott A. MacDiarmid, MD, FRCPSC, Bladder Control and Pelvic Pain Center, Alliance Urology Specialists, Greensboro, North Carolina, Clinical Assistant Professor, Department of Urology, The University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, Jennifer Miles-Thomas, MD, FPMRS, Chesapeake Pelvic Health Center, Urology of Virginia, Virginia Beach, Virginia, Assistant Professor of Urology, Eastern Virginia Medical School, Norfolk, Virginia, Diane Newman, DNP, FAAN, Co-Director, Penn Center for Continence and Pelvic Health Division of Urology, University of Pennsylvania Medical Center, Philadelphia, Pennsylvania, Thomas Roth, PhD, Director of Sleep Disorders and Research Center, Henry Ford Hospital, Detroit, Michigan
Written by: Hanna Stambakio, BS, Clinical Research Coordinator, Division of Urology,, University of Pennsylvania, @PennUrology at the 2018 AUA Annual Meeting - May 18 - 21, 2018 – San Francisco, CA USA


