(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Urbano Anido Herranz discussing real-world experience study from seven Galician medical centers using radium-223 for mCRPC. Radium-223 represents an option for symptomatic mCRPC after demonstrated an overall survival improvement in the ALSYMPCA trial.1 However, radium-223 real-life experiences are scarce, particularly after the results of the ERA-223 trial.2 The objective of this study was to describe radium-223 outcomes in real life practice, and specifically those related with bone health, sequence of treatment, and prognostic factors.
This was a multicenter retrospective cohort study of 146 patients with mCRPC treated with radium-223 in either the second line or third line of therapy or beyond in the context of routine clinical practice between March 11, 2013 and December 21, 2022, in seven Galician hospitals. Survival estimates were calculated by the Kaplan-Meier method, and groups were compared with the log-rank test. The Cox proportional hazards regression model was used to evaluate factors independently associated with overall survival. The median follow-up of 9.57 months, and all patients signed an informed consent form agreeing to participate in this observational study.
The baseline patient and disease characteristics are summarized in the following table:
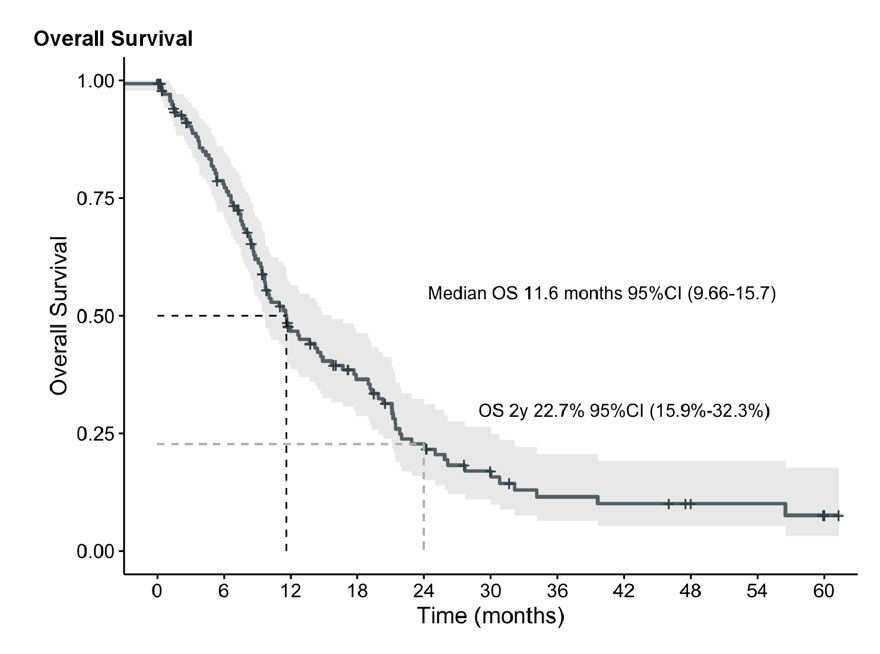
The median overall survival was 12 months (95% CI 9.7 – 16.0), and the best overall survival outcomes were achieved in second and third line: 18 months (95% CI 12.0 – 26.0) and 9.4 months (95% CI 8.0 – 12.0) months, respectively:
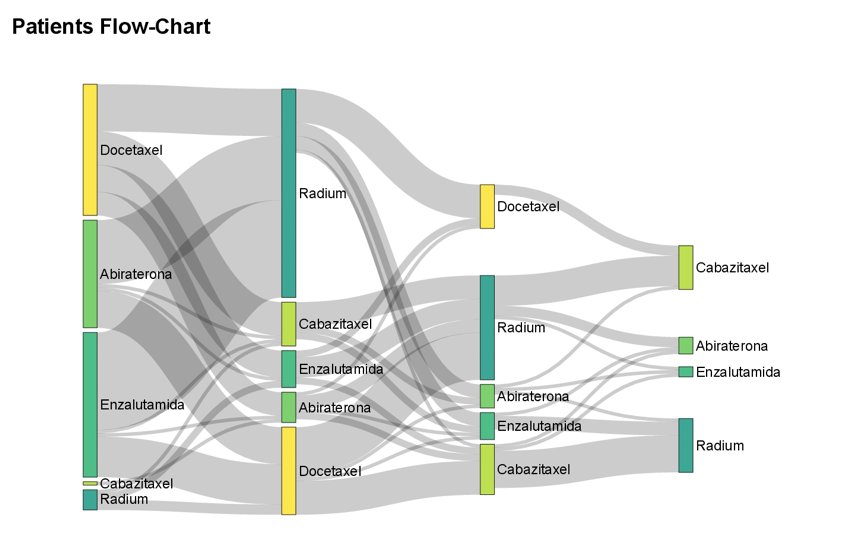
Among the baseline clinic-analytical factors analyzed, only alkaline phosphatase level >354 UI/L was correlated with a worse overall survival (HR 2.56, 95% CI 1.39 – 4.72) and the number of cycles of radium-223 received (HR 0.63, 95% CI 0.54 – 0.73) were prognostic factors for radium-223. Importantly, 99 patients (80%) were treated with bone-targeted therapy for bone metastases with no overall survival differences (p=0.66). The most frequent sequence of treatment was enzalutamide to radium (29 patients), with 62 patients treated with radium in the second line, as demonstrated in the Sankey diagram:
Dr. Herranz concluded this presentation discussing real-world experience study from 7 Galician medical centers using radium-223 for mCRPC with the following take-home messages:
- In this study, radium-223 efficacy was consistent with previously reported
- Best efficacy was achieved in the second or third line of therapy, independently of docetaxel use
- Patients with high baseline alkaline phosphatase had worse overall survival
- Few patients presented skeletal-related events, which could be explained by an adequate use of bone-targeted therapy (zoledronic acid or denosumab)
Presented by: Urbano Anido Herranz, MD, Area Sanitaria de Santiago de Compostela e Barbanza, Barbanza, Galicia
Co-Authors: Ovidio Fernandez Calvo, Sara Martinez Breijo, Natalia Fernández Núñez, Zulema Nogareda Seoane, Miguel Garrido Pumar, Javier Casas, Gloria Muñiz Garcia, Paula Portela Pereira, Antonio Gomez Caamaño, Daniel Pérez Fentes, Lucía Santomé, Gerardo Huidobro Vence, Aurea Molina Díaz, Ana Medina, Juan Ruiz Bañobre, Sergio Vázquez Estévez
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.
References:
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013;369(3):213-223.
- Smith M, Parker C, Saad F, et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019 Mar;20(3):408-419.


