(UroToday.com) The 2023 GU ASCO annual meeting included a session on prostate cancer, featuring a presentation by Dr. Ali Arafa discussing the impact of F-18 PSMA PET imaging on clinical decision-making in prostate cancer across disease states. Piflufolastat F-18 (18F-DCFPyL) PSMA PET imaging was recently approved by the FDA for initial staging, biochemical recurrence, and restaging of metastatic prostate cancer. Given that the PSMA PET is a next-generation novel imaging modality that carries a higher sensitivity for detecting metastatic disease compared with conventional imaging, Dr. Arafa and colleagues aimed to assess how its integration into clinical care may have impacted the management of patients.
This study identified 235 patients who underwent an 18F-DCFPyL PET scan at the University of Minnesota between August 2021 to June 2022. The median PSA at the time of imaging was 1.8 ng/ml (range: 0 - 3740 ng/ml). All PSMA PET scans were evaluated by an experienced nuclear medicine physician. Descriptive statistics were used to analyze its impact on the clinical care of 157 patients: 22 for initial staging, 109 with biochemical recurrence, and 26 patients with known metastatic disease. The remaining 78 patients had no information available to assess clinical utility.
Metastatic PSMA-avid lesions were detected in 152 of 235 (64.6%) patients. In patients undergoing initial staging of high-risk prostate cancer, 17 of 40 (42.5%) patients had metastatic lesions outside the prostate involving 10 regional and 5 non-regional lymph nodes, 1 visceral site, and 10 bone metastases. Overall, 17 of 40 (42.5%) scans were unremarkable, and 6 of 40 (15%) scans had equivocal scan results. For the patients with available clinical information, 12 of 22 (54.5%) had a change in their treatment plan post-DCFPyL, while 10 of 22 (45.5%) had no change:
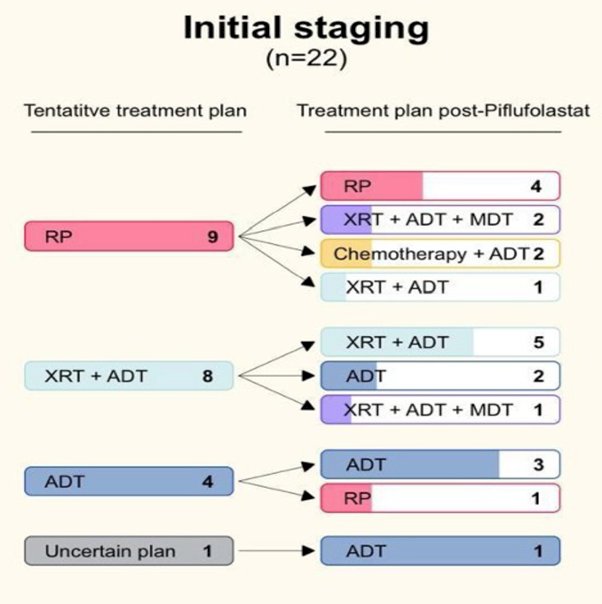
In the biochemical recurrence cohort, 93 of 151 (61.6%) had positive lesions: 28 local recurrences, 47 regional and 22 non-regional lymph nodes, 5 visceral and 28 bone metastases. Equivocal and negative scans accounted for 13 of 151 (8.6%) and 45 of 151 (29.8%) cases, respectively. There were 44 of 110 (40%) patients with clinical information that had a change in their treatment plan, while the plan did not change for 66 of 110 (60%) cases:
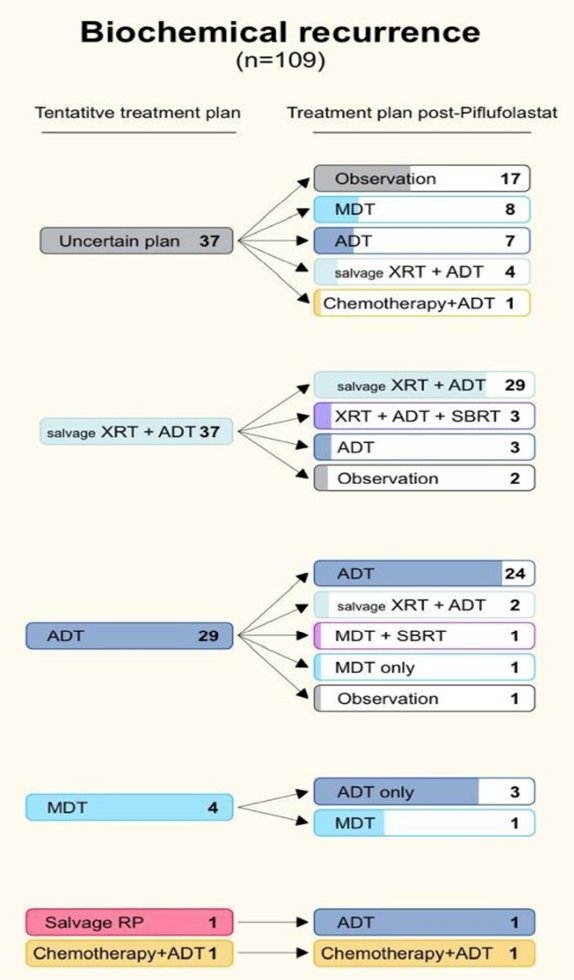
In patients with known metastatic prostate cancer undergoing restaging, 42 of 45 (93.3%) had DCFPyL-positive lesions; 12 local recurrences, 20 regional and 16 non-regional, 9 visceral, and 29 bone metastases. Equivocal and negative scans accounted for 2 of 45 (4.4%) and 1 of 45 (2.2%) of cases, respectively. Only 11 of 26 (42.3%) of those with clinical information had their tentative treatment plan adjusted post-DCFPyL. No change in the treatment plan was made in 15 of 26 (57.7%) cases:
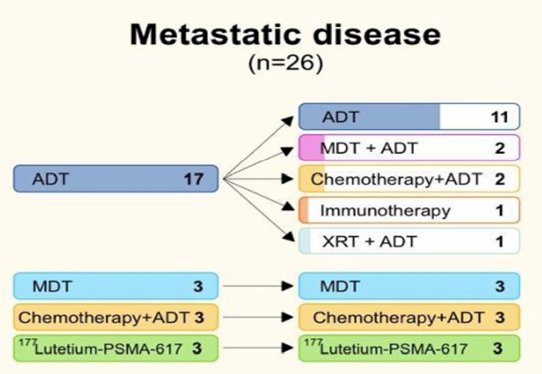
Dr. Arafa concluded his presentation discussing the impact of F-18 PSMA PET imaging on clinical decision-making in prostate cancer across disease states with the following concluding messages:
- Integration of the F-18 PSMA PET imaging substantially impacted clinical decision-making, especially for initial staging of high-risk localized disease (treatment change in 55%) and for biochemical recurrence (treatment change in 40%)
- It remains to be seen if this translates into superior survival outcomes
Presented by: Ali Arafa, MD, University of Minnesota, Minneapolis, MN
Co-Authors: Aditya Jain, Brad Humphrey, Jerry Froelich, Emmanuel S. Antonarakis
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, Thurs, Feb 16 – Sat, Feb 18, 2023.


