During the session on Targeted Therapy in the Context of Low-Volume Metastatic Prostate Cancer, Dr. Richard Lee offered some guidance to answer these questions.
Since this is currently an extremely broad field of research, Dr. Lee chose to focus more narrowly on fluciclovine and PSMA PET in patients with biochemical recurrence after curative-intent therapy for prostate cancer.
He began with an audience poll which demonstrated that 84% of members present at the meeting believe that next-generation imaging techniques such as these will eventually be integrated into the standard of care to the benefit of patients and most already have some access to these technologies. He then pointed out that 18F-fluciclovine PET is now available in >1000 centers in the US despite relatively recent approval by the FDA in May 2016.
He then went on to highlight the increased sensitivity of fluciclovine PET compared to conventional imaging, which in one study was 77.4% vs 18.9% in the overall cohort and 37.5% vs 12.5% in men with prostate-specific antigen (PSA) <1.0.1 He then showed data from Dr. Gerald Andriole’s LOCATE trial, 57% of biochemically recurrent men (with a median PSA of 1.00) were shown to have a positive lesion on fluciclovine PET, and the result of the test altered management of the patient in 59% of cases.
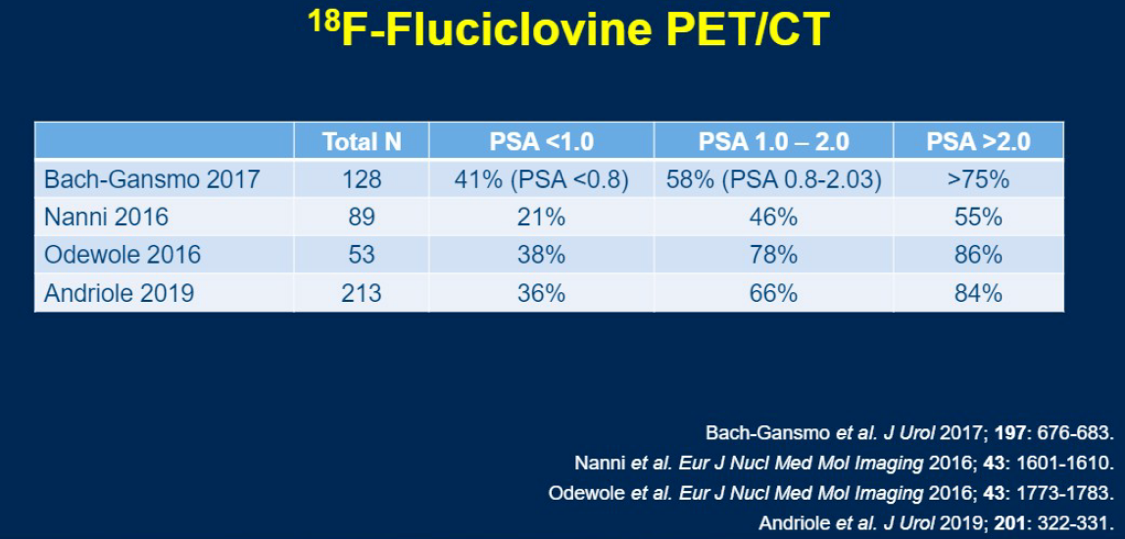
He then provided a nice summary table of the detection rate of fluciclovine PET across various PSA levels across several different trials. Briefly, for PSA <1.0, detection rates range from 21-21%, for 1.0-2.0 rates range from 46-78%, and for >2.0 rates range from 55-86%.
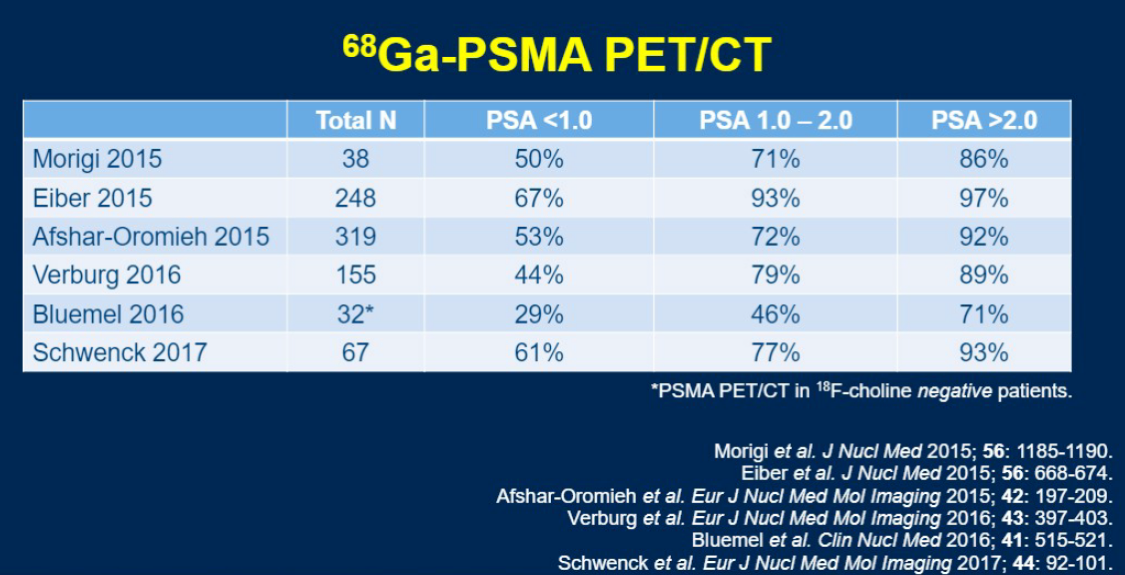
He then went on to discuss PSMA PET. PSMA is a transmembrane protein which is expressed in the 90-95% of prostate cancer cases. Antibodies to PSMA are usually bound to 68Ga, which allows for imaging of PSMA-rich areas, but has the disadvantage of a halflife of 68 minutes. Therefore while there currently exist many 68Ga formulations for targeting PSMA, several 18F formulations have been developed and may ultimately prove more useful clinically.
Multiple single-arm trials have demonstrated similar performance characteristics with PSMA as with fluciclovine, and one study demonstrated a similar rate of the study results changing management as was shown with fluciclovine (43%).2 However, the one study which directly compared the two modalities demonstrated significantly higher detection rates with PSMA over fluciclovine (56 vs 26% overall, 30% vs 8% in pelvic nodes, and 16% vs 0% in extrapelvic nodes).3
Dr. Lee also touched briefly on the idea that PSMA-targeted antibodies may in the future be bound to active antitumor agents and used for theranostics, however, he showed no data regarding the current efficacy of such therapy.
Finally, Dr. Lee concluded with a reminder that while these technologies have seem to clearly increase our sensitivity for the detection of metastatic prostate cancer, the trials around which our current guidelines are built relied on conventional imaging for metastatic evaluation. The interpretation of the results of next-generation imaging and the implications of these results for optimal medical management, therefore, is a very large unknown that will need to be addressed in the coming years.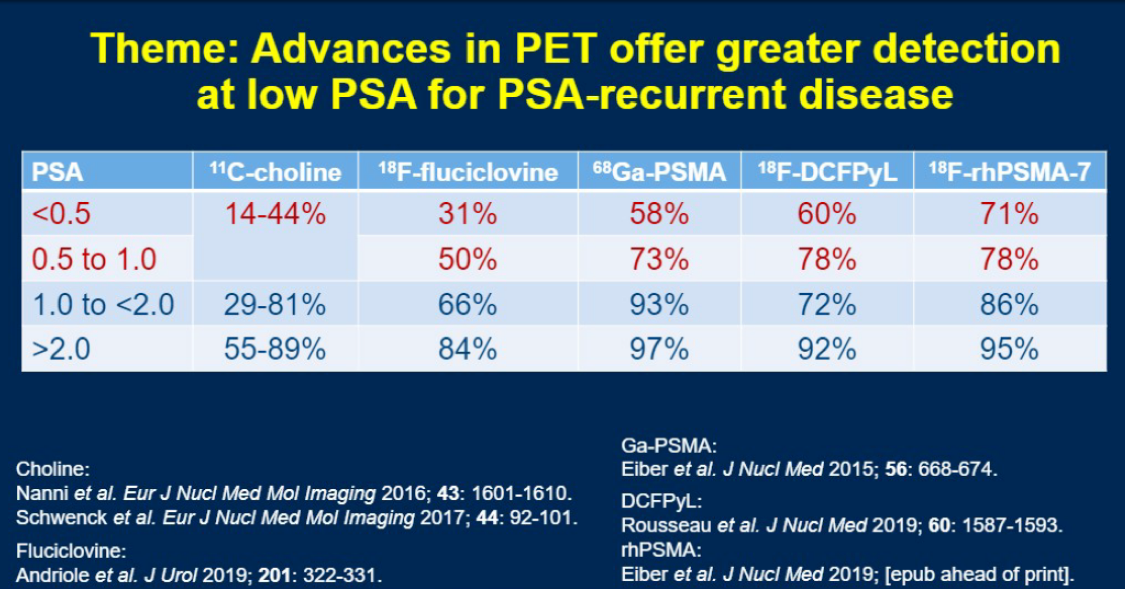
Presented by: Richard Lee, MD, PhD, Assistant Physician, Department of Medicine, Massachusetts General Hospital Cancer Center, Boston, Massachusetts
Written by: Marshall Strother, MD, Society for Urologic Oncology Fellow, Division of Urologic Oncology, Fox Chase Cancer Center, Philadelphia, Pennsylvania at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
1. Odewole, Oluwaseun A., Funmilayo I. Tade, Peter T. Nieh, Bital Savir-Baruch, Ashesh B. Jani, Viraj A. Master, Peter J. Rossi et al. "Recurrent prostate cancer detection with anti-3-[18 F] FACBC PET/CT: comparison with CT." European journal of nuclear medicine and molecular imaging 43, no. 10 (2016): 1773-1783.
2. Liu, Wei, Katherine Zukotynski, Louise Emmett, Hans T. Chung, Peter Chung, Robert Wolfson, Irina Rachinsky et al. "A Prospective Study of 18F-DCFPyL PSMA PET/CT Restaging in Recurrent Prostate Cancer following Primary External Beam Radiotherapy or Brachytherapy." International Journal of Radiation Oncology* Biology* Physics 106, no. 3 (2020): 546-555.
3. Calais, Jeremie, Francesco Ceci, Matthias Eiber, Thomas A. Hope, Michael S. Hofman, Christoph Rischpler, Tore Bach-Gansmo et al. "18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial." The Lancet Oncology 20, no. 9 (2019): 1286-1294.


