San Francisco, California (UroToday.com) During the Rapid Abstract Session A: Prostate Cancer session at the Annual ASCO GU 2020 meeting in San Francisco, CA, Dr. Karim Fizazi presented results from the analysis evaluating changes in pain and health-related quality of life associated (HRQL) with cabazitaxel (CBZ) and androgen-signaling-targeted inhibitor (ARTA) during the CARD study. The CARD study was a multicenter, randomized, open-label study, and reported superior radiographic progression-free survival and overall survival with CBZ vs. abiraterone or enzalutamide in patients with mCRPC who progressed on docetaxel and within 12 months on a previous alternative ARTA.
The objective of this study was to evaluate pre-planned endpoints affecting the quality of life in patients receiving CBZ vs. abiraterone or enzalutamide by measuring:
- Pain response and time to pain progression
- Symptomatic skeletal events
- Patient-reported outcomes (FACT-P)
Pain response was defined as a decrease > 30% from baseline in BPI-SF pain intensity score with no increased analgesic use. HRQL was assessed using the Functional Assessment of Cancer Therapy-Prostate (FACT-P) questionnaire. Patients were evaluable if they had received at least one dose of CBZ or ARTA and had a baseline FACT-P score plus at least one subsequent FACT-P measurement. A clinically meaningful improvement or deterioration of the total FACT-P score was defined as a difference of +/- 10 points from baseline. Clinically meaningful changes in HRQL and pain responses were confirmed at two consecutive evaluations ≥ three weeks apart during the on-treatment period.
Dr. Fizazi then summarized the results of this study. Of the 255 patients randomized, 172 (67.5%) had moderate to severe pain at randomization. Pain response and HRQL were evaluable for 111 (86.0%) and 108 (83.7%) for CBZ, and 109 (86.5%) and 114 (90.5%) for ARTA. The pain response was 45.9% vs. 19.3% for CBZ vs. ARTA. The probability of not having pain progression after 12 months was 66.2% vs. 45.3% CBZ vs. ARTA.
Improvement in total FACT-P score from baseline was reported by 27 (25.0%) patients vs. 26 (22.8%) for CBZ vs. ARTA. FACT-P score was maintained or improved for 81 (75.0%) patients with CBZ and 86 (75.4%) patients with ARTA. A deterioration in FACT-P from baseline was reported by 22.2% with CBZ vs. 24.6% with ARTA. The median time to FACT-P deterioration was 14.8 months for CBZ vs. 8.9 months for ARTA. The median time to first symptomatic skeletal events (SSE) was not resulted in CBZ and was 16.7 months for ARTA with a hazard ratio of 0.59.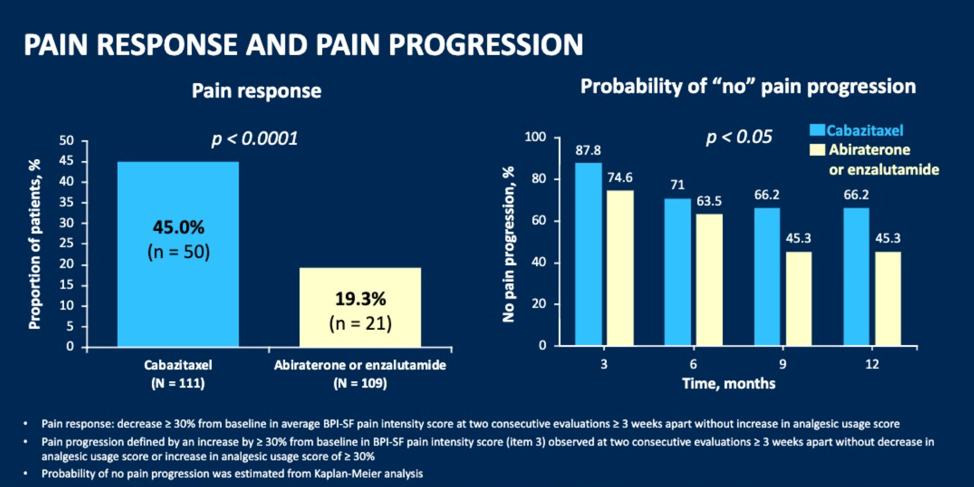
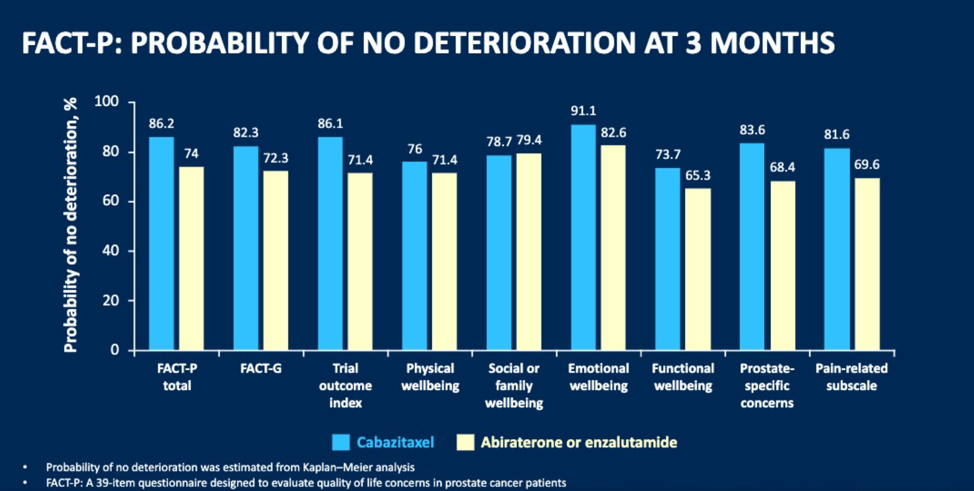
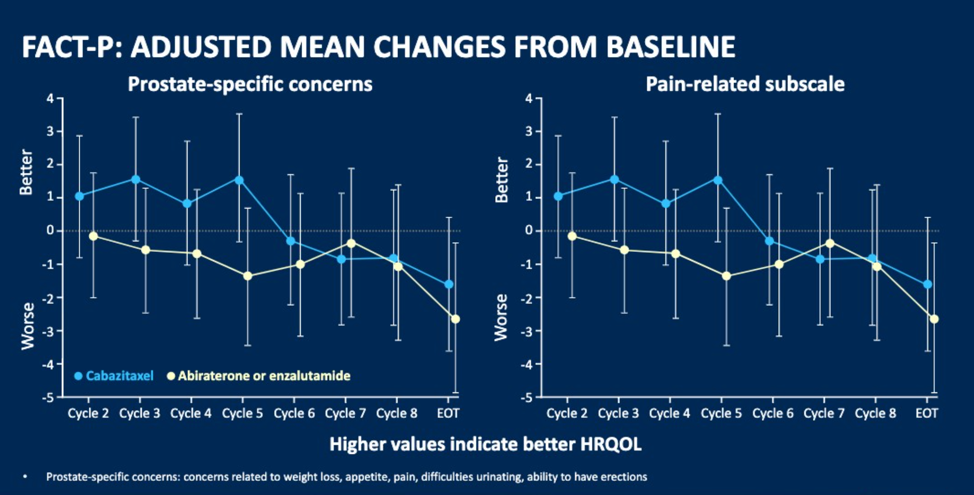
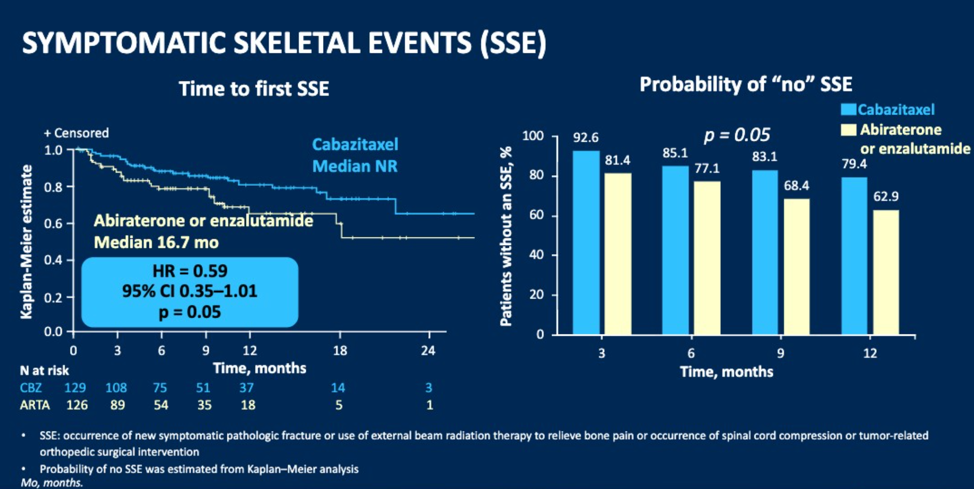
Dr. Fizazi concluded his presentation with a summary that CBZ improved pain, time to pain progression, and time to SSEs. Changes in HRQL domains numerically favored CBZ. These results support the use of CBZ over ARTA as a standard of care in patients previously treated with docetaxel and who progressed within 12 months of treatment with the alternative ARTA.
Presented by: Karim Fizazi, MD, PhD, Head of the Department of Cancer Medicine, Institut Gustave Roussy, Villejuif, France and Professor of Oncology at the University of Paris.
Written by: Abhishek Srivastava, MD, Society of Urologic Oncology Fellow, Fox Chase Cancer Center, Fox Chase Cancer Center, Philadelphia, PA Twitter: @shekabhishek at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California


