(UroToday.com) The 2022 ASCO annual meeting featured a session on kidney and bladder cancer, including a presentation by Dr. Kelly Fitzgerald discussing progression-free survival after second line of therapy (PFS-2) for metastatic clear cell renal cell carcinoma (RCC) in patients treated with first-line immunotherapy combinations. Front-line therapy with immunotherapy combinations is the standard of care for metastatic clear cell RCC, with ipilimumab/nivolumab (IO/IO) and several combinations of a VEGFR-targeted tyrosine kinase inhibitor with a PD-1 inhibitor (TKI/IO) showing superior efficacy to TKI monotherapy. PFS-2 evaluates the ability to be salvaged by 2nd line therapy and is a surrogate for overall survival (OS). In this study, PFS-2 was compared in patients receiving 1st line IO/IO vs TKI/IO for metastatic clear cell RCC.
This was a retrospective analysis performed on patients with clear cell RCC treated at Memorial Sloan Kettering Cancer Center between January 1, 2014 and December 30, 2020, in cohorts defined by 1st line: IO/IO or TKI/IO. PFS-2 is defined as time from start of 1st line to progression on next therapy, or death. Patients without a PFS-2 event were censored at a prespecified cutoff date. The objective response rate to 1st (ORR1st) and 2nd (ORR2nd) lines were compared with Fisher’s exact test. OS, PFS-2, and time on therapy are estimated with the Kaplan-Meier method and compared with the log-rank test.
One hundred seventy-three patients received 1st line IO/IO (n = 90) or 1st line TKI/IO (n = 83):
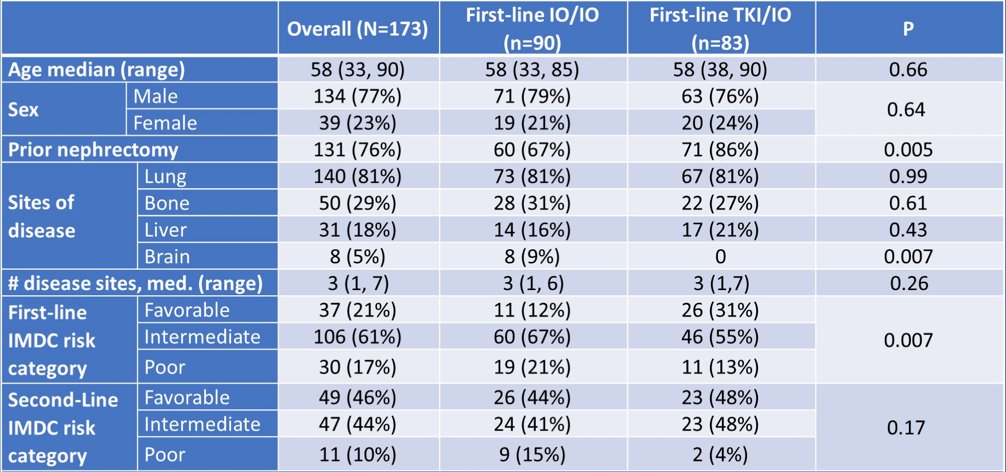
There were 52 patients receiving IO/IO and 40 patients receiving TKI/IO that had a PFS-2 event. 1st line TKI/IO regimens included: 34% axitinib + pembrolizumab, 29% lenvatinib + pembrolizumab, 25% axitinib + avelumab, 11% other. Of note, more IO/IO patients had brain metastases and intermediate/poor MSKCC risk category (respectively p = 0.007, p < 0.001). ORR1st and median months on 1st line were higher with TKI/IO vs IO/IO (65% vs 39%, p < 0.001; 16.1 vs 5.1, p < 0.001). ORR2nd was higher with IO/IO vs TKI/IO (47% vs 13%, p < 0.001), and median months on 2nd line was not significantly different (7.7 vs 7.1, p = 0.30). Median PFS-2 for TKI/IO was 44 months (95% CI 27, 53) vs 23 months (95% CI 16, 47) for IO/IO (p = 0.13):
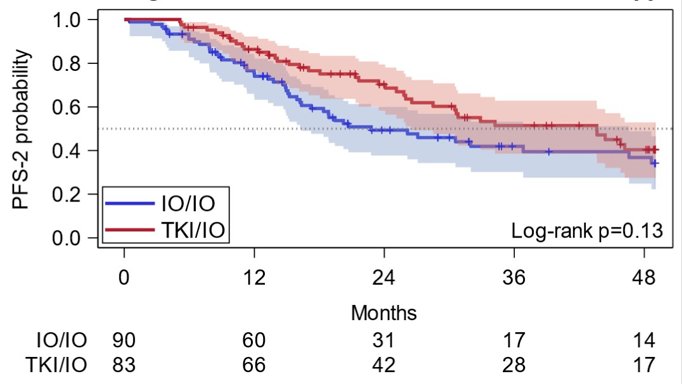
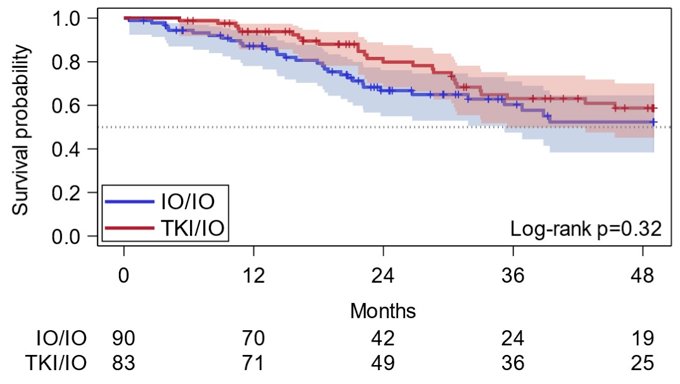
For TKI/IO and IO/IO groups, respective PFS-2 at 12 months was 86% (95% CI 77, 92) and 74% (95% CI 63, 82). PFS-2 at 36 months was 51% (95% CI 39, 63) and 42% (95% CI 30, 53). OS was not significantly different (p = 0.32; 3-year OS: IO/IO 60%, 95% CI 47, 71; TKI/IO 62%, 95% CI 49, 73) between the two groups:
Dr. Fitzgerald concluded this presentation by discussing progression-free survival after PFS-2 for metastatic clear cell RCC in patients treated with first-line immunotherapy combinations:
- There is no significant difference in PFS-2 between patients who received first-line IO/IO versus TKI/IO combinations
- First-line ORR in this real-world cohort is in close concordance with ORR reported in clinical trial cohorts
- Currently employed clinical stratification methods for assigning IO/IO versus TKI/IO are appropriate until a biomarker predicting IO/IO versus TKI/IO responders is available
Presented by: Kelly N. Fitzgerald, MD, PhD, Memorial Sloan Kettering Cancer Center, New York, NY,
Co-Authors: Cihan Duzgol, Andrea Knezevic, Natalie Shapnik, Ritesh Kotecha, David Henry Aggen, Maria Isabel Carlo, Neil J. Shah, Martin H Voss, Darren R. Feldman, Robert J. Motzer, Chung-Han Lee
Affiliations: Memorial Sloan Kettering Cancer Center, New York, NY, Columbia University Medical Center, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


