To briefly summarize, the UNISON trial enrolled patients with advanced nccRCC with good performance status (ECOG 0/1). Patients were initially treated with nivolumab monotherapy. Salvage therapy with the combination of ipilimumab (1mg/kg) + nivolumab (3mg/kg) every 3 weeks for up to 4 doses were offered to the 41 patients with disease refractory to nivolumab. Patients with response to the salvage combination therapy were offered ongoing nivolumab for up to 1 year. The UNISON trial was powered to distinguish a clinically relevant improvement in objective tumour response rate (OTRR) from 15% to 30% in people taking ipilimumab and nivolumab in part 2.
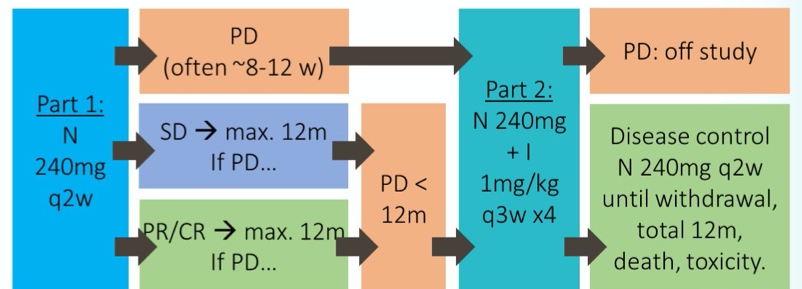
The UNISON trial enrolled 85 patients who received nivolumab monotherapy. Following monotherapy, 41 patients were refractory to nivolumab and were well enough to receive combination therapy. These 41 patients had a representative spectrum of nccRCC histologies (n=41; papillary 44%, chromophobe 20%, Xp11 translocation 12%, RCC unclassified 7%, other 17%). In terms of salvage therapy, patients received a median of 3 doses and the median time on treatment was 2.1 months. Subsequent to salvage therapy, the median follow-up at the time of reporting was 20.3 months.
The objective tumour response rate of salvage nivolumab and ipilimumab among those refractory to nivolumab monotherapy was 10% with 1 complete and 3 partial responses. Further, stable disease was experienced by 36% of pts and disease progression by 52%. Overall, the 6- month disease control rate was 45% (95% CI: 34%, 56%) and the 6-month and 12-month progression-free survival (PFS) was 25% (95% CI: 13-39) and 14%. The median PFS was 2.6 months (95% CI: 2.2, 3.8). Only 14% of patients were free of progression at 12 months.
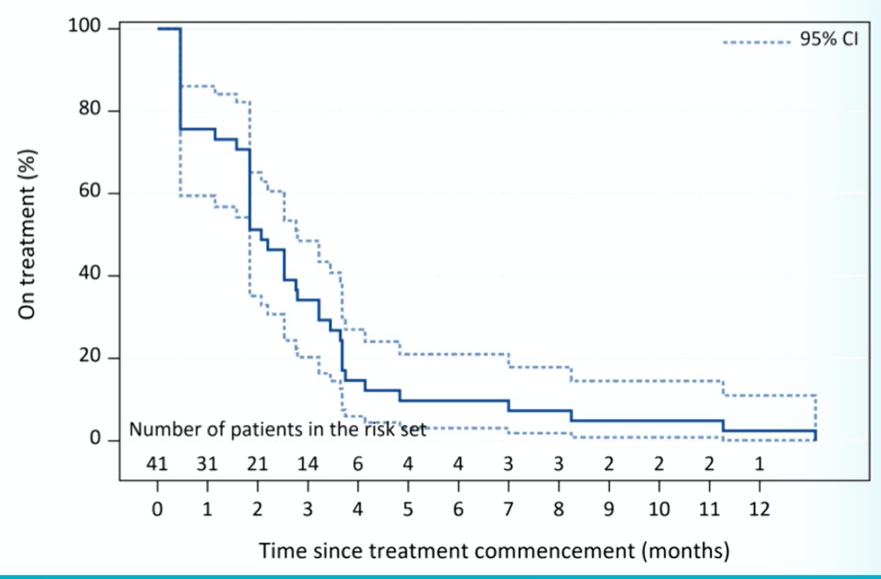
The median number of doses was 3. Most patients discontinued therapy within 3 months with a median time to treatment discontinuation of 2.1 months.
The toxicity of combination nivolumab and ipilimumab was similar to previous reports, including CheckMate 214, with 68% of patients experiencing serious adverse events and 34% experiencing treatment-related serious adverse events.
The authors concluded that the UNISON trial failed to meet its primary endpoint: a minority of patients with nccRCC refractory to nivolumab derive benefit from combination nivolumab and ipilimumab.
Presented by: Craig Gedye, FRACP, MBChB, PhD, Calvary Mater Newcastle, Waratah, NSW, Australia
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


