In the Poster Discussion section of the 2020 American Society of Clinical Oncology Virtual Annual Meeting, Dr. Small and colleagues presented updated overall survival data assessing apalutamide in non-metastatic castration-resistant prostate cancer from SPARTAN.
The methodology of the SPARTAN trial has been previously described in both presentations and publications. In short, 1207 patients with non-metastatic castration-resistant prostate cancer and a prostate-specific antigen doubling time of ≤ 10 months were randomized in a 2:1 fashion to apalutamide 240mg daily or placebo, in addition to continuing androgen deprivation therapy.
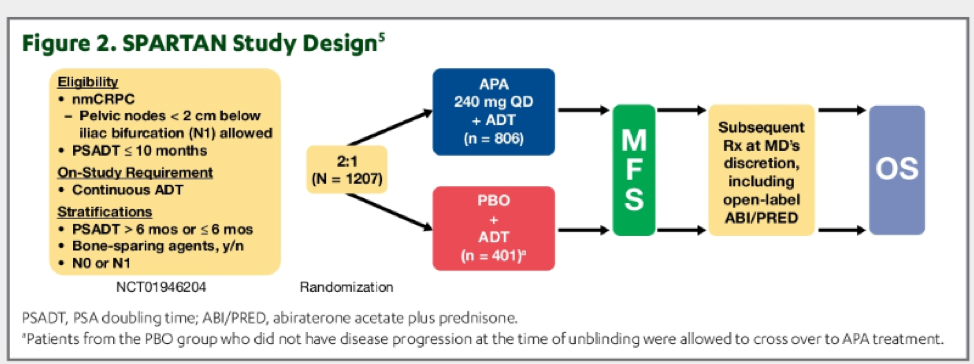
As previously published, SPARTAN demonstrated a significant improvement in metastasis-free survival and a statistically non-significant improvement in overall survival. As a result of meeting the primary outcome endpoint, patients in the SPARTAN trial were unblinded and patients randomized to placebo who had not progressed were offered apalutamide cross-over. After initial analysis meeting the primary outcome of metastasis-free survival, 76 patients receiving placebo crossed-over and receiving apalutamide. At the time of progression, patients could receive open-label sponsor-provided abiraterone acetate + prednisone, or other anti-neoplastic therapies.
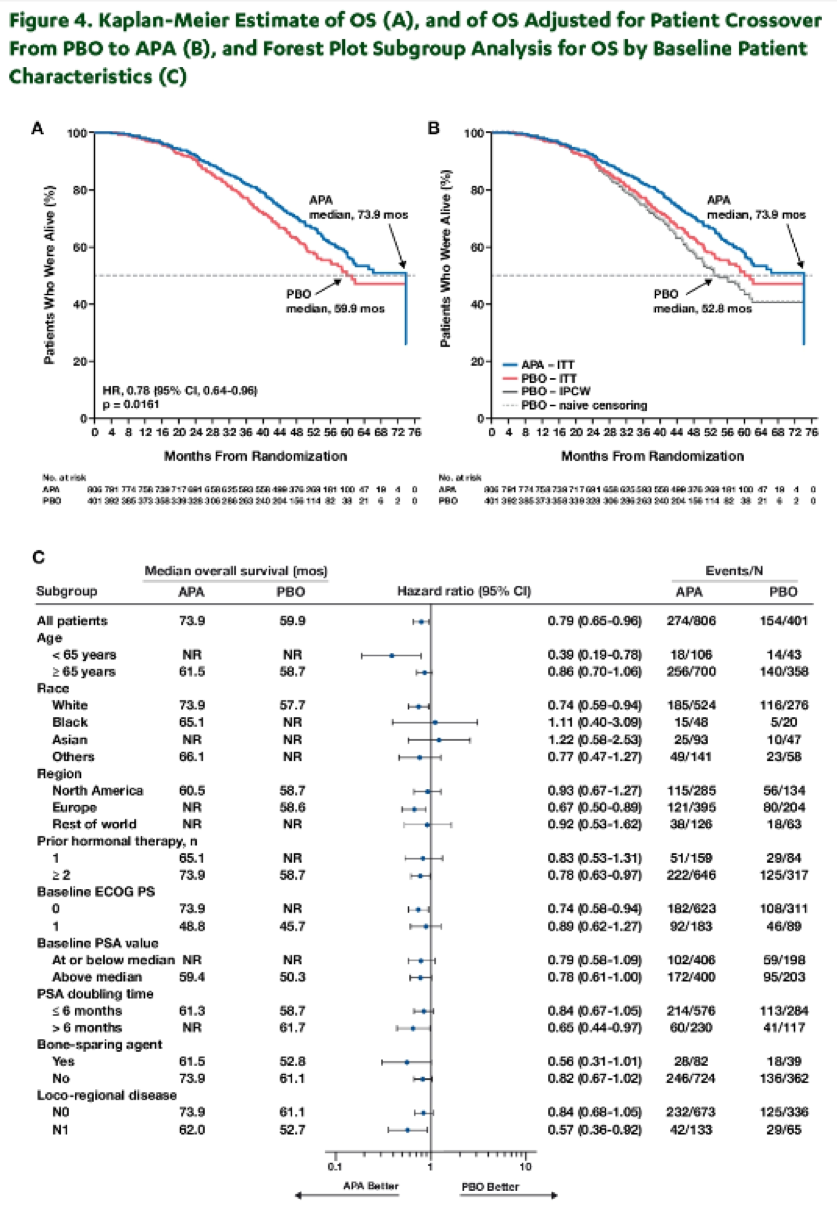
Overall survival and time to cytotoxic chemotherapy were tested by group sequential testing procedure with O’Brien-Fleming (OBF)-type alpha spending function. Median overall survival was assessed using the Kaplan Meier technique and associated hazard ratios, with 95% confidence intervals, were calculated using Cox proportional hazard models. A sensitivity analysis for overall survival, accounting for crossover using a naïve censoring approach, was conducted.
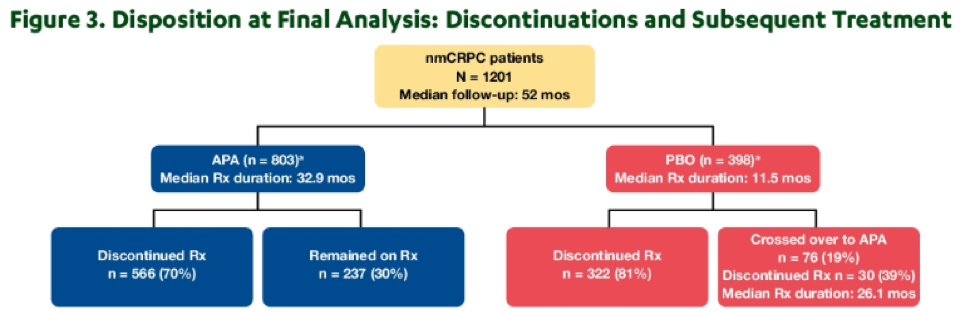
Median follow-up at the time of data cut-off was 52 months. At this time, 428 (of a required 427) deaths had occurred to allow for analysis. As would be expected from the previously published analysis of the primary outcome (metastasis-free survival), median duration of treatment was longer for patients receiving apalutamide (32.9 months) compared to those receiving placebo (11.5 months).
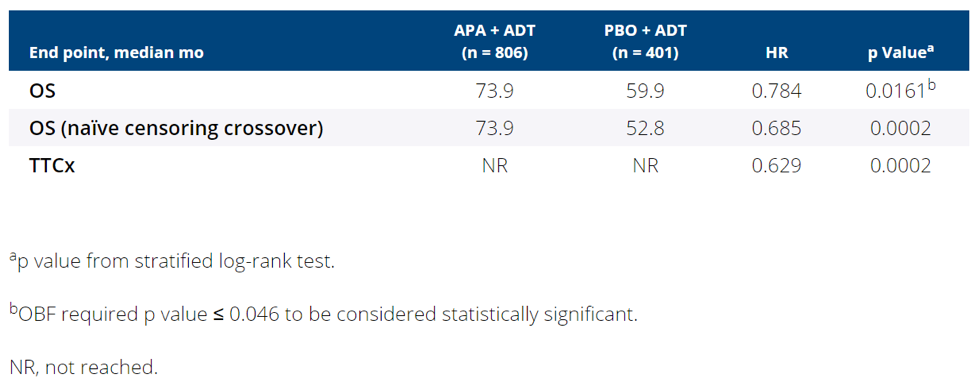
Median overall survival was significantly longer among men receiving apalutamide than placebo (73.9 versus 59.9 months), corresponding to a relative reduction of 21.6% in the risk of death (hazard ratio 0.784, p-value 0.0161; OBF threshold of 0.046 for statistical significance). Results were similar in the sensitivity analysis accounting for cross-over and when assessing time to cytotoxic chemotherapy (Table).
The adverse event profile at this analysis was consistent with the previous reports from the SPARTAN cohort.
Thus, this third and final analysis of the SPARTAN cohort demonstrates a statistically significant improvement in overall survival for patients receiving apalutamide for non-metastatic castration-resistant prostate cancer.
Presented by: Eric Jay Small, MD, FASCO, Professor of Medicine, Department of Medicine/Division of Hematology/Oncology, UCSF Helen Diller Family Comprehensive Cancer Center, San Francisco, CA.
Co-Authors: Fred Saad, Simon Chowdhury, Stephane Oudard, Boris A. Hadaschik, Julie N Graff, David Olmos, Paul N. Mainwaring, Ji Youl Lee, Hiroji Uemura, Peter De Porre, Andressa Smith, Sabine Doris Brookman-May, Susan Li, Ke Zhang, Oliver Brendan Rooney, Angela Lopez-Gitlitz, Matthew Raymond Smith
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2020 ASCO Annual Meeting, Virtual Scientific Program #ASCO20, May 29-31, 2020.
Related Content:
View: Changing the Landscape of nmCRPC - The SPARTAN Trial - Eric Small
View: Prolonging Time to Metastatic Disease, The SPARTAN Study - Matthew Smith


