However, the introduction of targeted therapies, including tyrosine kinase inhibitors (TKI) and mTOR inhibitors, have drastically changed the outcomes of mRCC patients. While not providing a cure, they are able to provide long-term response in some patients. In doing so, they have significantly extended the survival of patients with mRCC. Unfortunately, with this advancement in systemic therapy, the need for cytoreductive nephrectomy has been called into question. Especially in patients with advanced RCC (intermediate and poor risk) who have high volume disease outside of the kidney, there has been increasing emphasis on providing systemic therapy up-front and avoiding delays by putting the patient through a major operation.
Indeed, population-level analyses have demonstrated that, in real-world practice, utilization of cytoreductive nephrectomy is quite low (Vaishampayan KCA 2017). So, while clinical trials still include CNx as a prerequisite for inclusion, this is not really happening in community practice.
Retrospective series and meta-analyses have continued to demonstrate a survival benefit to CNx in the setting of mRCC. (Heng et al. EU 2014, Bhindi et al. J Urol 2018) However, this has primarily been in IMDC favorable or intermediate risk patients (IMDC 1-3 risk factors).
Mejean noted that there has been general acceptance that for a surgically fit patient with good performance status and low-volume metastases, nephrectomy makes sense. Similarly, he notes that most agreed that for patients with poor PS (2+) and high volume metastatic disease, upfront systemic therapy made sense. It is for the patients in between, PS 0-1, intermediate volume metastatic disease, for whom nephrectomy was uncertain. However, this lack of equipoise likely contributed to their poor accrual, as seen below.
Level 1 data has long been lacking. However, that is quickly changing.
In SURTIME, an EORTC sponsored randomized control trial, patients were randomized to sunitinib followed by CNx (deferred CNx arm) and subsequent sunitinib versus upfront CNx followed by sunitinib. SURTIME closed early in 2016 due to poor accrual (likely due to difficulty enrollment criteria) – and was likely underpowered. SURTIME results were presented at ESMO 2017. On intent to treat analysis, deferred CNx was non-inferior to up front CNx – HR 0.57 favoring deferred nephrectomy (p=0.032). However, as they did not meet their sample size requirements, the study was technically underpowered. Yet, the data suggests that deferring CNx was likely not detrimental in targeted therapy era.
The authors of another Phase III randomized trial assessing the utility and timing of CNx in the treatment paradigm of mRCC in conjunction with TKI’s report their results. CARMENA is a French randomized clinical trial comparing patients who were randomized to either CNx and sunitinib versus sunitinib alone without CNx; patients were recruited primarily from France, but also the UK.
It had difficulty accruing patients – they expected a sample size of 576 patients (and 456 events) to demonstrate non inferiority hypothesis, with 80% power at a 1-sided significance level of 5%. Yet, ultimately, only 450 patients were included – so it should be kept in mind that the study is underpowered.
- There were 2 planned interim analysis: after 152 events and 302 events
- The study was stopped after the 2nd interim analysis as the independent DSMB felt the primary objective had been met
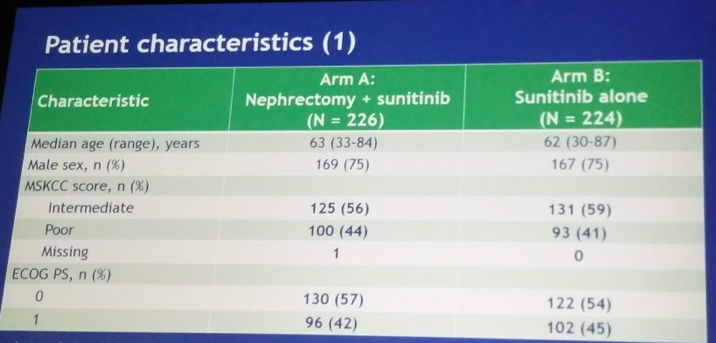
Demographics of the cohort(s) are as follows:
- Median age was 62
- ECOG-PS was 0 in 56% and 1 in 44%
- MSKCC risk groups were intermediate/poor in 55.6/44.4% (arm A) and in 58.5/41.5% (arm B)
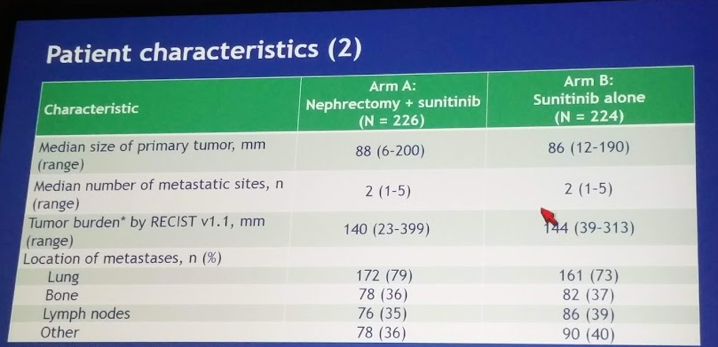
- Median primary tumor size ~8 cm, median total tumor burden ~14 cm in both arms
Study design: Pts were randomized 1:1 to either CN followed by sunitinib (arm A) or sunitinib alone (arm B), and stratified by MSKCC risk groups. Sunitinib was given at 50 mg/d, 4/6wk with dose adaptation to routine practice. In arm A, sunitinib had to start 4 to 6 weeks after surgery.
Study protocol is detailed below:
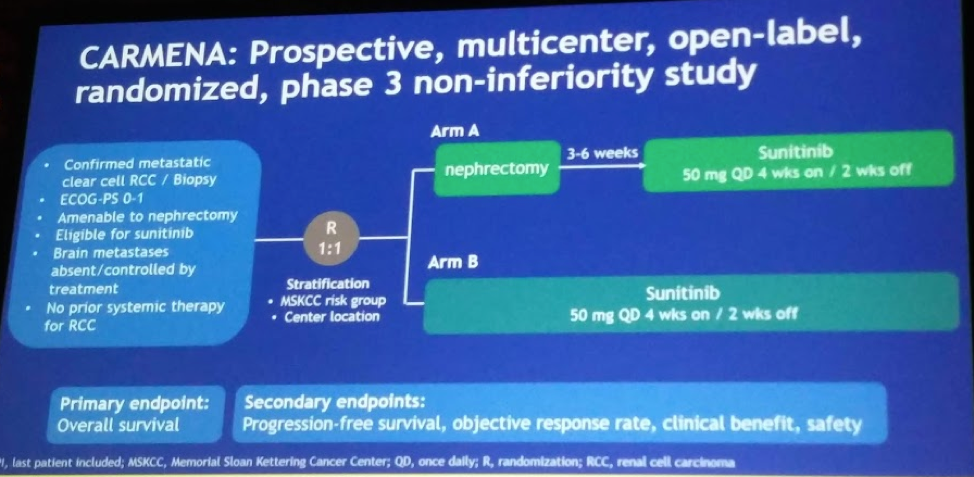
The primary endpoint was overall survival (OS). Secondary outcomes were: PFS, ORR, Clinical benefit, Safety
In terms of adherence to trial protocol, in arm A, 6.7% did not have CN and 22.5% never received sunitinib; in arm B, 4.9 % never received sunitinib and 17% had secondary nephrectomy (delayed nephrectomy).
The breakdown of the population is seen below:
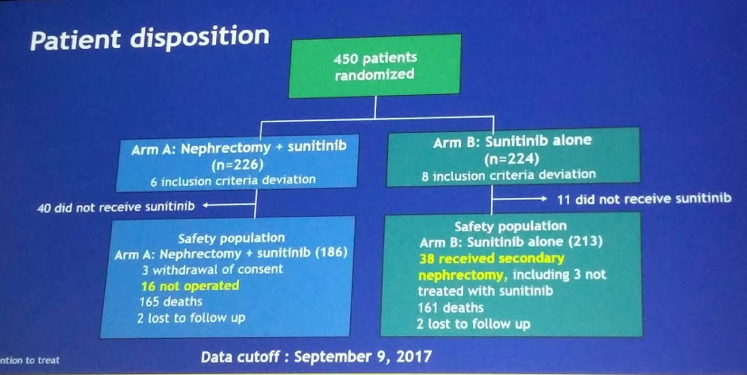
In terms of analysis, there were 3 analyses:
1. Intent-to-treat: All 226 in Arm A and 224 in Arm B
2. Patient Population 1: Patients who received nephrectomy in Arm A (N=205) and patients who received sunitinib in Arm B (N=206)
3. Patient Population 2: Patients who received nephrectomy + sunitinib in Arm A (N=176) and patients who received sunitinib in Arm B (N=206)
At the time of the analysis, 326 deaths have been observed with a median follow-up of 50.9 months. In terms of OS for the ITT population, median OS for arm A was 13.9 months and for Arm B was 18.4 months (HR 0.89, 95%CI 0.89-1.10, non-inferior). The table below highlights the OS and ORR differences between the arms - no difference in response rate and PFS (HR 0.82, CI 0.67-1.00) was observed.
However, he did note that “clinical benefit”, or disease control beyond 12 weeks, was significantly better in Arm B than in Arm A (47.9% vs. 36.6%, respectively, p = 0.022).
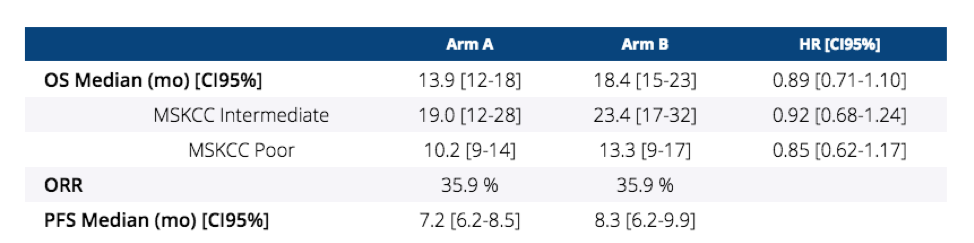
When looking at the subpopulations:
1. Median OS – HR similar to ITT population, but wider confidence intervals
2. PFS - HR similar to ITT population, but wider confidence intervals
Adverse event profile was as expected for sunitinib.
Mortality for nephrectomy was minimal (4 deaths, 2%). Most complications were Clavian-Dindo Grade 1-2. 16% were Grade 3-4.
Secondary nephrectomy in Arm B – 38 patients (17%) had secondary nephrectomy. Of these, 7 (18.9%) were due to symptoms and considered emergent. However, in 30 patients who had strong response to systemic therapy, they underwent elective nephrectomy. Median 11.1 months from time of randomization.
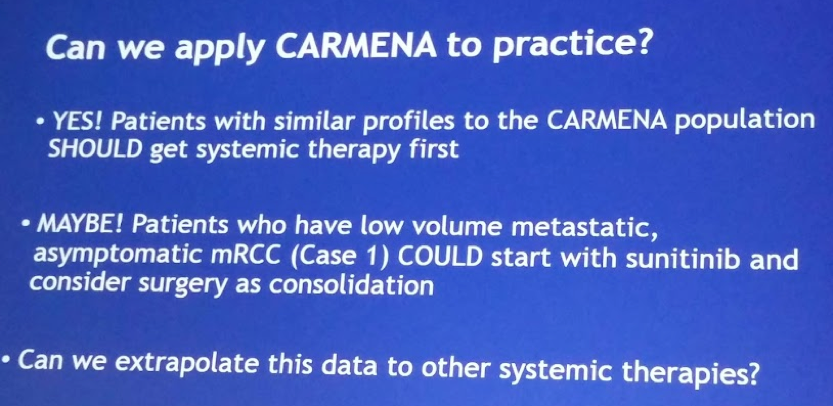
Based on these results, despite its limitation, CARMENA suggests that sunitinib alone is not inferior to CNx followed by sunitinib in synchronous mRCC both in intermediate and poor MSKCC risk groups. Favorable risk patients were intentionally not included. This confirms the findings of SURTIME and on the weight of these two randomized controlled trials, CNx should not be anymore the standard of care when medical treatment is required.
Limitations:
1. CARMENA does not include favorable risk mRCC
2. CARMENA, just like SURTIME, did not reach its expected sample size, so its results are underpowered – however, with more patients, its results favoring Arm B may have been significant!
DISCUSSION
Daniel George, MD from Duke provided an excellent follow-up to Mejean’s presentation. Below are some of his key points:
1. Lack of equipoise amongst medical oncologists and urologists led to poor accrual – and the significant preponderance of poor risk patients in the study. Fully 40% or more of both arms were poor risk patients, likely due to hesitation by providers to sent favorable or even low-volume intermediate risk patients for accrual; probably because they already felt these patients would benefit from nephrectomy.
2. Cytoreductive nephrectomy, by nature, functions as a debulking surgery. Yet, the primary tumor made up only about ~60% of the tumor burden in these patients. This patient population had a large volume of extra-renal disease, making it less likely they would benefit from debulking.
3. Interpretation of non-inferiority – he put up an important slide, which notes that while the ITT and PP1 populations met the non-inferiority endpoint, the PP2 analysis (which compares patients in Arm A who had both nephrectomy and sunitinib to patients in Arm B who had sunitinib) did not.
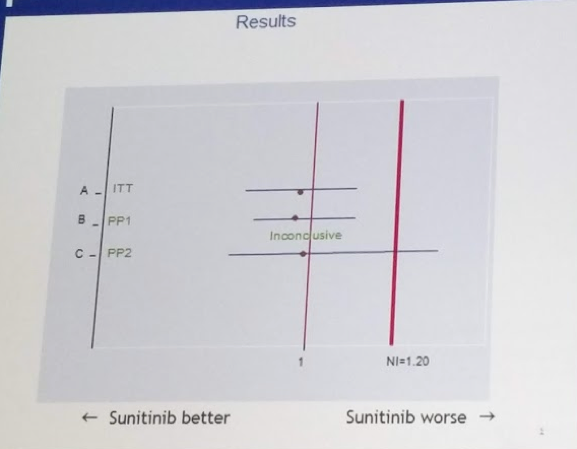
His caveat to this is that, as a clinician, he is inclined to follow the ITT analysis – as we cannot in real life predict who will make it through nephrectomy to systemic therapy.
4. Recent advances in first-line therapy for intermediate and poor-risk patients (CheckMate 214 and CABOSUN) have displaced sunitinib as first line therapy for these patients; Nivo/Ipi and cabozantinib are preferred. We are not certain what the impact of these changes have on the role of nephrectomy in this population.
His take-home slides are great! See below:

Presented by: Arnaud Mejean, MD, Ph.D., Department of Urology, Hôpital Européen Georges-Pompidou - Paris Descartes University
Discussion Presented by: Daniel George, MD, Professor of Medicine and Surgery, Divisions of Medical Oncology and Urology in the Duke University School of Medicine.
Co-Authors: Bernard Escudier, Simon Thezenas, Jean-Baptiste Beauval, Lionnel Geoffroy, Karim Bensalah, Antoine Thiery-Vuillemin, Luc Cormier, Herve Lang, Laurent Guy, Gwenaelle Gravis, Frederic Rolland, Claude Linassier, Marc Olivier Timsit, Laurence Albiges, Christian Beisland, Michael Aitchison, Sandra Colas, Thierry Lebret, Alain Ravaud; Department of Urology, Hôpital Européen Georges-Pompidou - Paris Descartes University, Paris, France; U1015 INSERM, Gustave Roussy Cancer Campus, Paris Saclay University, Villejuif, France; Institut du Cancer de Montpellier (ICM), Univ Montpellier, Montpellier, France; Department of Urology, CHU Rangueil, Toulouse University, Toulouse, France; Department of Medical Oncology, Institut de Cancerologie Lorraine-Alexis Vautrin, Vandoeuvre les Nancy, Vandoeuvre Les Nancy, France; University Hospital Pontchaillou, Rennes, France; Medical Oncology, CHU Besancon, Besançon, France; Department of Urology, Hopital Francois Mitterrand, CHU Dijon, Dijon, France; Department of Urology, CHU Strasbourg, Strasbourg University, Strasbourg, France; Hopital Gabriel Montpied CHU, Clermont-Ferrand, France; Medical Oncology, Institut Paoli-Calmettes, Marseille, France; Department of Medical Oncology, Institut de Cancérologie de l'Ouest, Nantes, France; CHU Bretonneau, Tours, France; Department of Urology, Hôpital européen Georges-Pompidou - Paris Descartes University, Paris, France; Medical Oncology, Gustave Roussy, Université Paris-Saclay, Villejuif, France, Villejuif, France; Department of Clinical Medicine, University of Bergen, Bergen, Norway; The Beatson West of Scotland Cancer Centre, Glasgow, United Kingdom; URC/CIC Paris Descartes Necker Cochin, Paris, France; Hôpital Foch, Suresnes, France; Department of Medical Oncology, Hôpital Saint-André, University of Bordeaux-CHU, Bordeaux, France
Written by: Thenappan Chandrasekar, MD, Clinical Fellow, University of Toronto, Twitter: @tchandra_uromd at the 2018 ASCO Annual Meeting - June 1-5, 2018 – Chicago, IL USA
Read the NEJM Abstract: Sunitinib Alone or after Nephrectomy in Metastatic Renal-Cell Carcinoma


