Exome sequencing discovered commonly mutated kidney cancer-related genes, including BAP1, SETD2, KDM5C, and KDM6A, which are involved in histone or microRNA production, in 3-12% of patients, 3-8% of patients, 1% of patients, and 21-41% of patients, respectively. In fact, epigenetic aberrations are frequently observed in RCC, demonstrating that changes in epigenetic modifications, such as aberrant microRNA production or promoter methylation, are crucial in the development of RCC because of changes in gene expression without changes in the genome's sequence. These changes may serve as both potential predictive biomarkers and medication development targets and may influence the prognosis of patients with advanced RCC.2
The capacity to alter gene expression in the tumor cell is the basis for the interest in creating novel medications in the field of epigenetics. Drugs that target epigenetics are still in the early stages of research in kidney cancer, although they may show promise in the near future. Histone deacetylase (HDAC) and DNA methyltransferase (DNMT) inhibitors have been investigated in RCC up to this point, however, unrepresentative clinical studies have had dismal outcomes. In this regard, several therapeutic combinations are being investigated since epigenetic therapy may be able to restore the susceptibility of tumors to anti-VEGF therapy.3
A stable condition for at least 6 months or a partial response was shown in 19% of patients in a phase I research assessing the efficacy of vorinostat, an HDAC inhibitor, in combination with pazopanib. The study's median PFS and median OS were 2.2 months and 8.9 months, respectively. Another clinical trial that included 33 evaluable patients and investigated the use of vorinostat and bevacizumab together reported overall response rates of 18%, median PFS of 5.7 months, and median OS of 13.9 months. Cytidine analogues, which are DNMT inhibitors, prevent DNMT action when they attach to DNA and cause its breakdown. These medications thereby cause DNA demethylation, the production of genes that have been silenced by promoter DNA methylation, and they encourage the death of tumor cells.
These medications, together with azacytydine and decitabine, are being studied in ccRCC clinical trials for haematological cancers. Patients receiving DNMT inhibitors in clinical trials experienced abrupt and widespread genome demethylation, which has been linked to off-target adverse outcomes. This demethylation may not only activate normally repressed genes but also restore improperly suppressed gene expression. Combining ICI with epigenetic treatment is a new paradigm in RCC. It might be a possible treatment strategy since it is thought that epigenetic abnormalities cause cancer cells to avoid the immune system. The capacity to reinstate immunological recognition by the host and immunogenicity serves as the foundation for this method. Several studies are still running evaluating this strategy. (Fig 1)
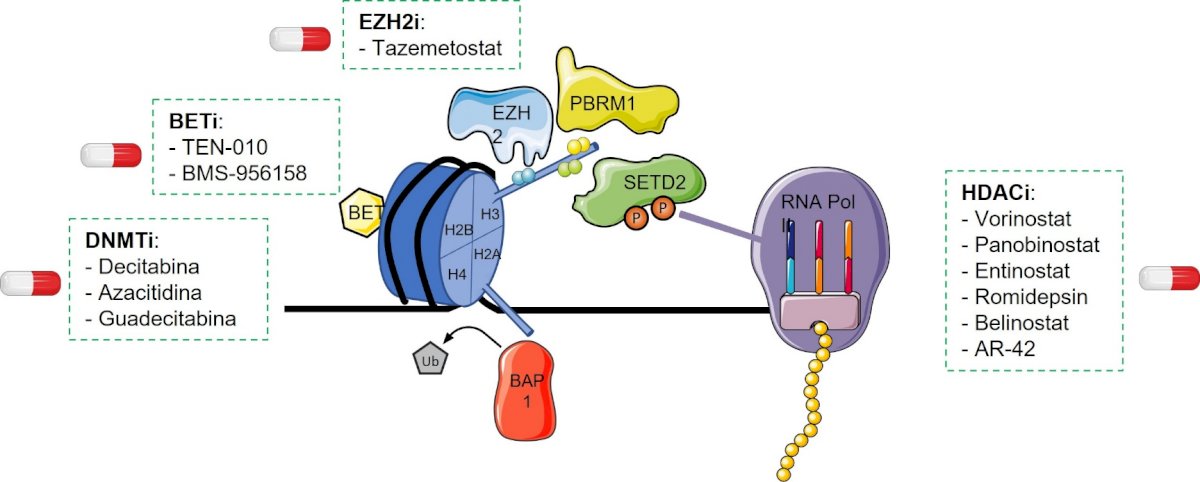
Another possible tactic relies on controlling oncogenic mi-RNAs by either activating downregulated mi-RNAs or silencing those implicated in RCC. However, the sole phase I study that evaluated the tumor suppressor miR-34 mimic MRX34 in ccRCC was halted due to significant immunologic side effects. Moreover, because unregulated long non-coding RNAs play a role in suppressing TSG, their function may be possibly reversed by EZH2 inhibitors. This is an intriguing prospective therapeutic method.
Epigenetic-based therapies have several restrictions when used in RCC. RCC is not the only condition where epigenetic changes occur. These epigenetic modifications affect both normal and malignant cells. Additionally, newly discovered medications activate other physiologically suppressed genes instead of specifically restoring the silence of genes that are crucial for the growth of tumors. However, because RCC is a heterogeneous tumor with several tumoral clones and genetic and epigenetic abnormalities, it is challenging to choose which epigenetic changes should be targeted.4
Epigenetic processes have evolved for the development of prospective biomarkers or treatment options based on epigenetics and have provided strong evidence of their function in the genesis and progression of RCC. Consequently, a number of studies are being conducted on RCC patients with encouraging outcomes, mostly as combination treatments.
Written by: Javier Molina-Cerrillo, Álvaro Ruiz, Javier Pozas, Teresa Alonso-Gordoa
- Medical Oncology Department, Hospital Universitario Ramón y Cajal. Medicine School, Alcalá University, Madrid, Spain.
- Matteo Santoni, Oncology Unit, Macerata Hospital, Macerata, Italy
- Francesco Massari, Medical Oncology, IRCCS Azienda Ospedaliero-Universitaria di Bologna, Bologna - Italia
- Ignacio Ortego, Enrique Grande (@drenriquegrande), Medical Oncology Department. MD Anderson Cancer Center Madrid, Madrid, Spain.
- Victoria Gómez, Medical Oncology Department, Hospital Universitario Ramón y Cajal. Medicine School, Alcalá University, Madrid, Spain.
- Sung H, Ferlay J, Siegel ME, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA: A Cancer Journal for Clinicians. 2021:71;209-249.
- Molina-Cerrillo J, Santoni M, Ruiz Á, et al. Epigenetics in advanced renal cell carcinoma: Potential new targets [published online ahead of print, 2022 Oct 17]. Crit Rev Oncol Hematol. 2022;180:103857.
- Joosten S, Smits K, Aarts M, et al. Epigenetics in renal cell cancer: mechanisms and clinical applications. Nature Reviews Urology. 2018;15:430–451.
- Angulo JC, Manini C, López JI, Pueyo A, Colás B, Ropero S. The Role of Epigenetics in the Progression of Clear Cell Renal Cell Carcinoma and the Basis for Future Epigenetic Treatments. Cancers. 2021; 13(9):2071.


