(UroToday.com) In a Hot Topic session of the Société Internationale D’Urologie (SIU) 2021 annual meeting focused on the role of artificial intelligence (AI) in urology, Dr. Mark Emberton discussed the role of AI in prostate cancer imaging. Building on his plenary session talk regarding advances in prostate cancer imaging over the past decade, Dr. Emberton discussed his perspectives on the changing landscape of imaging in the coming decade.
He began by highlighting currently utilized multiparametric magnetic resonance imaging (mpMRI) as interpreted by expert clinicians. The human brain, he emphasized, is quite good at spatial orientation and the identification of particular characteristics. He highlighted an example of a prostate mpMRI that demonstrated an abnormality in the mid-line of the peripheral zone, an unusual location for a tumor focus.
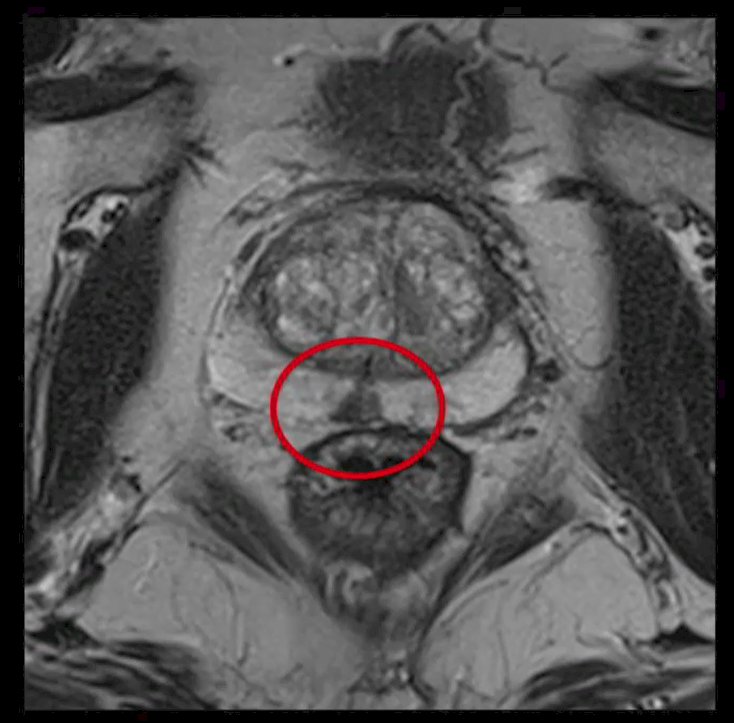
The question for this session is whether a machine learning approach can appreciate this lesion with the speed and accuracy of a clinician. While most of the literature focuses on mpMRI, he emphasized that we should think broadly about the ability of imaging approaches to identify a range of clinical phenotypes based on combinations of imaging results. For example, he highlighted work with PSMA and mpMRI derived phenotypes. The use of these two approaches can allow for four pair-wise based categories of tumors. These phenotypes, with ongoing work, will hopefully allow for derivation of prognostic and predictive information. However, he emphasized that these characteristics may also be appreciable in other modalities, including micro-ultrasound. Artificial intelligence-driven post-processing may allow for the derivation of further details from available images.
Dr. Emberton emphasized that, based on available techniques, we have potentially maximized the available information and clinical utility of mpMRI. One of the most relevant questions he highlighted is whether we can obtain relevant information with the omission of dynamic contrast-enhanced sequences. These DCE sequences are the most timely and costly components of a multi-parametric MRI. Thus, omission, if still able to provide clinically relevant and actionable information, would make MRI much more accessible. Additionally, a simpler scan would lend itself more naturally to assessment with artificial intelligence due to the ability to generate pixel or voxel based heat maps.
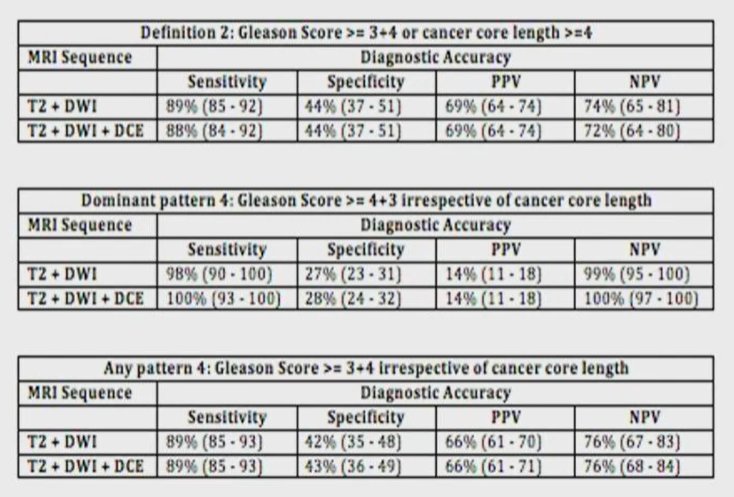
He then highlighted a relatively recent systematic review comparing bi-parametric with multi-parametric MRI. Among 10 studies assessing 1705 patients with 3419 lesions, the area under the ROC curve was 0.88 for mpMRI and 0.89 for bi-parametric MRI (p=0.994) suggesting that these approaches were roughly equivalent. In particular, he noted that often the DCE sequences are often poorly performed and thus may not contribute significantly to the interpretation of the overall study. However, there is a paucity of data assessing this question prospectively. In a post hoc analysis of the PROMIS data which prospectively assigned scores to each sequence, he described the relative value of two sequences (T2 + diffusion weighted) versus three sequences (T2 + diffusion weighted + dynamic contrast enhanced). When a variety of different outcome definitions were used, the sensitivity, specificity, positive predictive value, and negative predictive value of bi-parametric and multi-parametric MRI were essentially equivalent.
He then discussed two prospective studies which are planned to assess whether it is safe to drop the gadolinium enhanced sequence from mpMRI. One of these utilizes a within-patient repeated measures design while the other is a non-inferiority randomized trial. However, he noted that a bi-parametric MRI is more likely to be compromised by artefact due to rectal gas.
In addition to these advances in mpMRI, he discussed alternative technological advances that may help by providing better diffusion sequences and exclusive T2 sequences. Much of this relates to post-processing which may be improved by the use of artificial intelligence. He cited one such example derived at UCL deemed VERDICT-MRI which seeks to better distinguish Gleason score, beyond the information available from the apparent diffusion coefficient (ADC). This approach generates an anatomic heat map that may be particularly well suited to an AI-based interrogation. However, moving beyond this, an ability to rely exclusively on T2 sequences would allow a substantially shorter scan time as well as avoid artefactual issues related to diffusion in the rectum. He highlighted an approach again developed at UCL deemed luminal water fraction which may be derived from the T2 sequence and correlates well with histology. Again, this approach generates heat maps which may be assessed with an automated analysis approach.
He highlighted additional approaches to understanding the underlying metabolic status of cancer, citing the example of carbon-13 imaging which may be overlayed with anatomical images. Currently, this is experimental rather than clinically relevant.
Based on current data, AI in prostate imaging is currently useful for calculating prostate volume with semi-automatic segmentation of the prostate contour driven by machine learning approaches and auto-segmentation of prostate zones through convolutional neural networks. Further, there is evidence that AI may assist with lesion detection, segmentation, and characterization. This may in fact improve detection of lesions in the transition zone and enhance the characterization of marginal lesions (PI-RADS 3) though this has not yet been adopted in routine practice. More interestingly, radiomics may allow for a prediction of aggressivity with discrimination of aggressive and non-aggressive lesions. Additionally, while imaging approaches are routinely used for loco-regional staging, there have been few studies assessing the role of AI in this context.
He emphasized that there is consistent and growing literature supporting the use of AI to improve prostate cancer diagnosis and risk stratification. Currently, while semi-automatic and auto-segmentation are in routine clinical practice, detection and characterization of lesions remain essentially experimental. Further, the literature on this topic is quite limited with most studies having small sample sizes, few addressing external validity or repeatability, and overall low quality. Assessing the question of AI accuracy, sensitivity, and specificity, he noted that while most studies show quite promising results in the derivation/training set, typically they perform considerably more poorly invalidation.
He cited a recent example that sought to identify, segment, and classify prostate tumors using AI with a radical prostatectomy gold standard. He noted that, even before training, there was substantial curation of the included patients and MRIs such that these results lack external validity. Using 92 characteristics, the authors sought to distinguish low grade (GGG1-2) from high grade (GGG3-5) tumors. Dr. Emberton suggested that this dichotomy is among the easiest discrimination task able to be addressed. In the end, 6 features were included in the algorithm and this performed reasonably well with an AUC = 0.81 in their validation subset with an overall accuracy of 75%.
In summary, he emphasized that imaging is likely to be simplified in the coming years. This will facilitate the integration of AI in imaging which will be expected, therefore, to have an increasing role in helping clinicians maximize the clinical utility of imaging studies.