(UroToday.com) In the second Hot Topics session of the Société Internationale D’Urologie (SIU) 2021 annual meeting focused on the role of prostate specific membrane antigen (PSMA) and theranostics in prostate cancer, Dr. Ugur Selek presented on the role of targeted salvage radiotherapy and metastasis-directed therapy in patients with biochemical recurrent disease.
To begin, he addressed the question of who benefits most from prostate directed radiotherapy, emphasizing that low volume metastatic disease is the target. Citing observational data published relatively recently based on registry data, primary prostate directed radiotherapy was not beneficial among all patients with metastatic disease. However, when stratified into M1a and M1b groups, a benefit of local prostate directed radiotherapy was seen in both groups. Dr. Selek emphasized important effect modification due to PSA: in each group, where the PSA was <10 ng/mL, there was a survival benefit to the use of local prostate directed radiotherapy. However, in those with PSA >10 ng/mL, no benefit was seen suggesting that the burden of disease modifies the benefit of local, prostate-directed radiotherapy. He, therefore, concluded that there is a benefit to local radiotherapy for patients with M1a and low-volume M1b.
Similarly, a post hoc analysis of the STAMPEDE trial demonstrated that there was both an overall survival and disease-free survival benefit to the use of local prostate-directed radiotherapy where patients had three or fewer bony metastases. However, as the number of metastatic sites (as identified on conventional imaging) increased, the benefit of prostate directed radiotherapy decreased. Thus, the benefit was maximized among patients with a single metastasis. However, those with M1a disease derived a similar benefit as those with a single bony metastatic site, with a 7% overall survival benefit and 22% disease-free survival benefit at 3-years with the use of prostate radiotherapy.
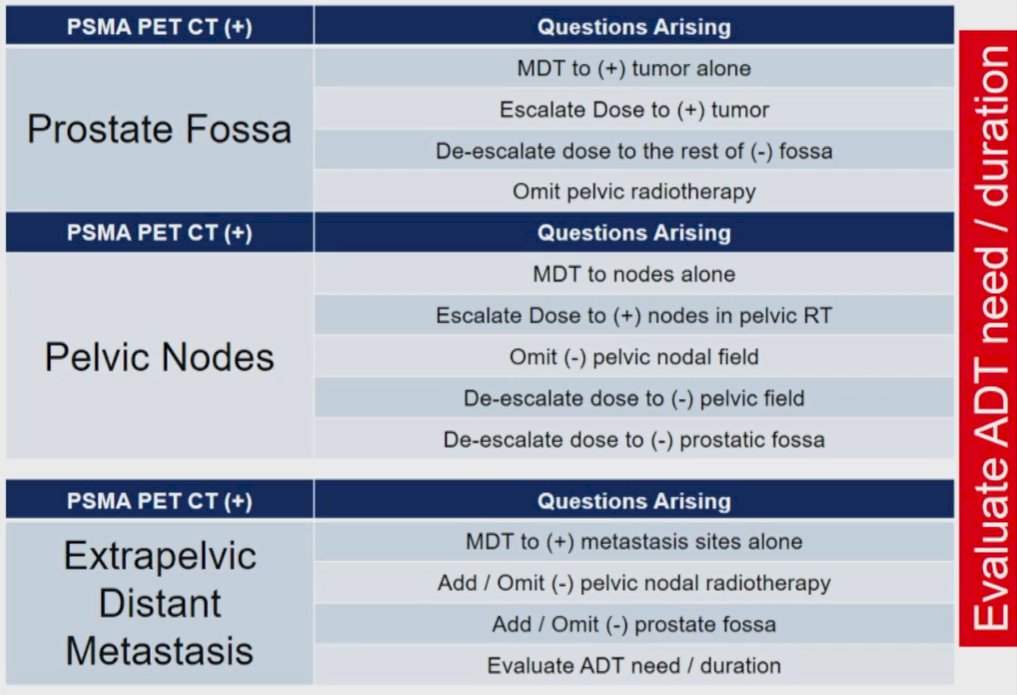
Moving from considering who benefits from radiotherapy to the prostate in the setting of metastatic disease, he then considered who benefits from metastasis directed radiotherapy in this setting. He first cited an ESTRO-ASTRO consensus document highlighting that oligometastatic disease is defined as 1-5 metastasis-directed therapy eligible lesions. These may be synchronous, metachronous, oligo-progressive, or oligo-persistent, in the basis of where they fall in the disease course and treatment trajectory. The argument for aggressive metastasis-directed therapy is predicated in part on the notion that metastatic sites may feed the progression of further metastasis. Further, relatively “indolent” metastatic lesions have the potential to transform and become aggressive foci of accelerated metastatic disease.
The SABR-COMET trial, led by Dr. Palma, examined the role of stereotactic ablative radiotherapy as metastasis-directed therapy in patients with oligometastatic cancer. While patients with prostate cancer comprised only a subset of the study (21%), overall, the results demonstrated improved overall survival for those receiving SABR.
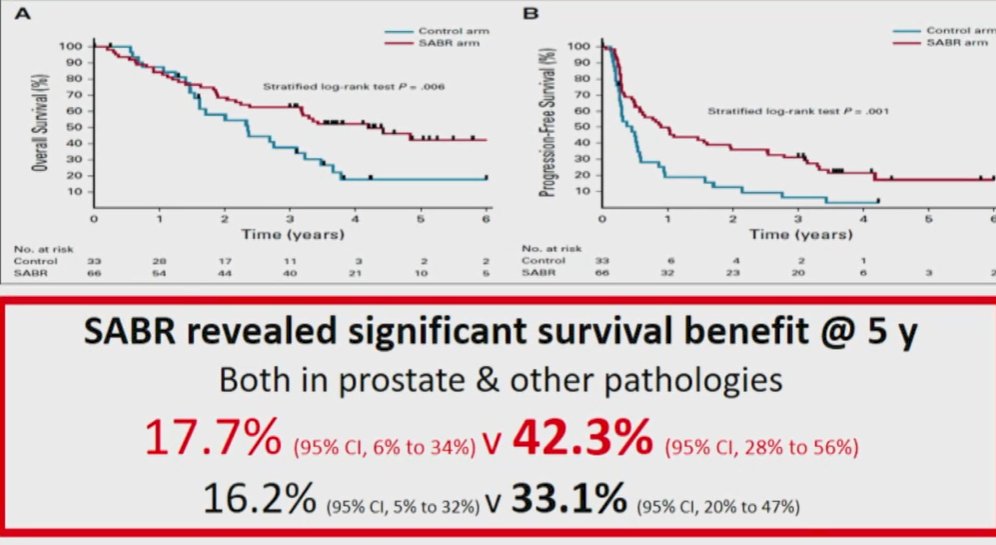
The STOMP trial, led by Dr. Ost, similarly assessed the role of metastasis directed therapy in patients with oligometastatic prostate cancer recurrence (staged by choline PET). In this randomized study, the use of metastasis-directed therapy led to a significant delay in the initiation of systemic therapy with androgen-deprivation therapy (21 months vs 13 months). The POPSTAR study similarly assessed the role of SABR to oligometastatic prostate cancer lesions (staged with 18F-NaF PET-CT) demonstrating that one-third of patients treated with SBRT were free of disease progression and the need for ADT at 2-years. Most recently, ORIOLE compared observation and SABR in a similar cohort of men with 1-3 metastasis based on conventional imaging, finding decreased rates of disease progression, according to the PCWG3 definition, at 6 months. Interestingly, nearly two-third of men had 18F-DCFPyL imaging to which the authors were blinded. Interestingly, total consolidation of PSMA-avid lesions was associated with a significantly decreased rate of developing new lesions at 6 months (16% vs 63%). Further, assessing the primary outcome, progression was less common in the MDT group.
Dr. Selek then considered the question as to whether changing management is a clinically significant outcome for assessing the value of targeted imaging in patients with prostate cancer. The LOCATE trial, utilizing 18F-fluciclovine, found that approximately half of patients (48%) had a change in the recommended management strategy when advanced imaging is used in the biochemical recurrence setting. In particular, there were notable changes to the radiotherapy approach planned. Similarly, prior analyses utilizing choline PET-CT demonstrated that the majority of patients with biochemical recurrence following prostatectomy recurred outside a standard post-prostatectomy radiotherapy field, with high rates of nodal metastatic disease.
The CONDOR trial, a phase III assessment of 18F-DCFPyL, demonstrated changes in treatment planning among 64% of patients with radiographic recurrence. Of these, the majority (79%) were attributable to positive findings, while the remainder were as the result of negative findings. Similar work from Australia led by Dr. Azad examining 68Ga-PSMA-PET demonstrated that, even with a median PSA of 0.68 ng/mL, PSMA-avid disease was identified in 56% of patients. Further, radiographic findings were more common as the PSA level increased. As a result of these findings, the planned management was changed in 30 of 70 patients (432%). Summarizing these data, Dr. Selek emphasized that prospective trials have shown that restaging with PET-CT at the time of biochemical recurrence following radical prostatectomy is associated with a change in management in 43 to 64% of patients.
Further, emphasizing a recent systemic review and meta-analysis by Dr. Perera and colleagues, he emphasized that PSMA-PET/CT is superior to choline PET/CT with a higher sensitivity (75% vs 51%) for nodal metastasis. A prior prospective analysis led by Dr. Calais has further demonstrated its superiority to 18F-fluciclovine PET/CT. Interestingly, recent work has shown that while EAU risk groups are able to stratify the absolute rates of metastatic disease among patients with biochemical recurrence, non-regional disease is sufficiently common that all groups warrant PSMA-PET/CT for staging.
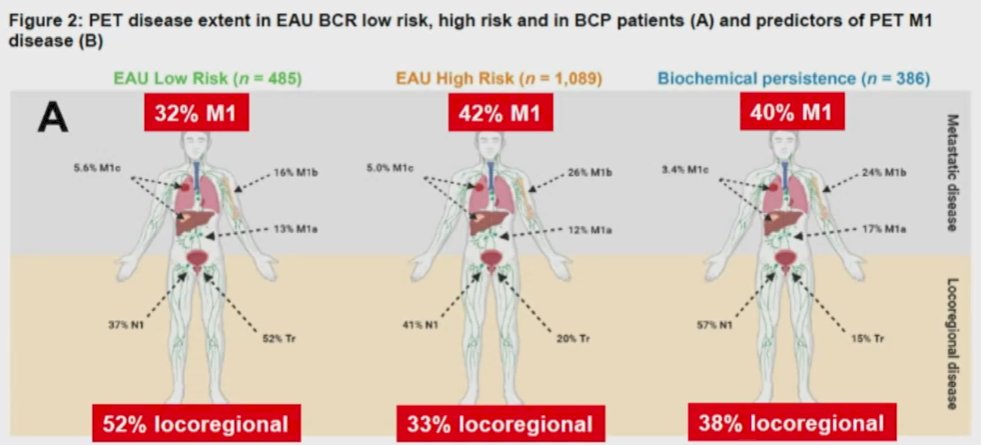
He then considered whether is it possible to properly salvage patients without the use of molecularly targeted agents. Examining data based on the use of choline PET/CT, approximately one-quarter of recurrences occur exclusively outside the pelvic, and another quarter includes both pelvic and extra pelvic disease. Thus, much of this disease would not be treated by standard radiotherapy fields.
Using a PSMA-PET-based nodal atlas to inform salvage therapy, 69% of positive lymph nodes at the time of recurrence would not be covered by the standard RTOG CTV. Additionally, for each 1 ng/mL increase in PSA, the risk of nodal metastasis outside the CTV increased by a factor of 1.43. These nodes may be found in the para-aortic, pararectal, paravesical, preacetabular, presacral, and inguinal regions.
Utilizing 68Ga-PSMA-11 and examining the question of early salvage treatment in patients with PSA levels <1 ng/mL, the use of PSMA-PET/CT identified lesions outside the standard radiotherapy field in 52 of 270 included patients. Further, they found that isolated local recurrence represents only 12% of those patients with biochemical recurrence after radical prostatectomy. These data formed the basis of a phase III trial that has completed enrollment, as of August 2020. This study will prospectively evaluate outcomes for patients with salvage treatment informed by PSMA-PET/CT or conventional imaging.
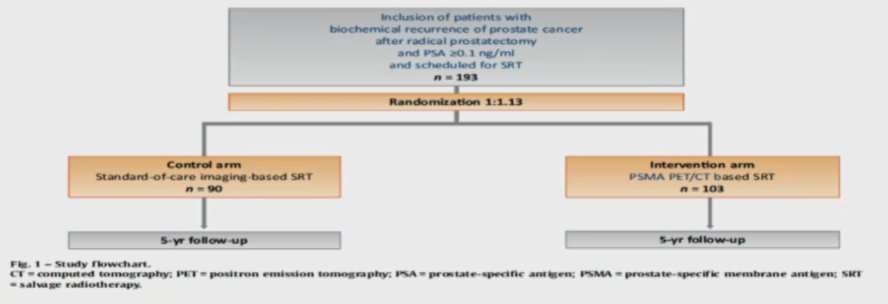
In this study, 9% of patients had disease outside the pelvis (M1 disease) while 20% had pelvic nodal disease with or without local prostate bed recurrence, and 13% had recurrence only in the prostate fossa.
Recurrence following prostate bed salvage radiotherapy may in fact be due to these distant sites of disease as has been shown in a number of analyses. Work using 18F-PSMA-PET/CT showed that, following radical prostatectomy and prostate-only radiotherapy, more than half of patients with failure had metastatic disease (53%). As a result, NRG/RTOG recently revised the consensus contouring guidelines for lymph node coverage in both primary treatment and post-operative settings for patients with prostate cancer. The furthers highlight that contouring should be based on information derived from PET imaging (whether PSMA or Axumin).
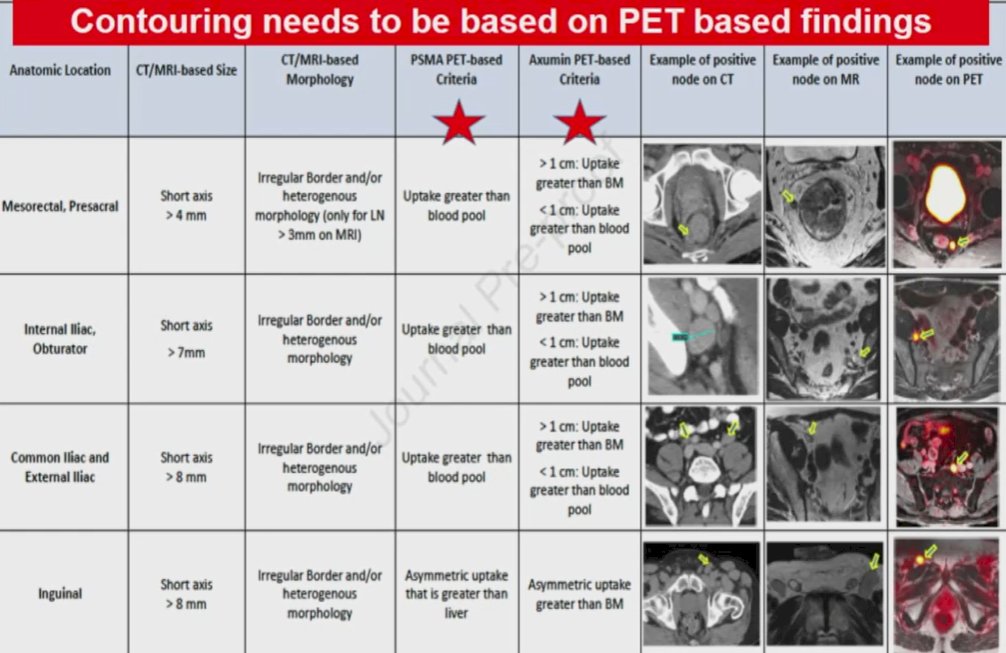
This approach was recently validated based on a secondary analysis of the LOCATE trial.
He then considered whether tailored treatment is feasible with the use of PSMA-PET/CT. Addressing this, he first cited data regarding the use of nodal SBRT from the pre-PSMA-PET/CT era. These data demonstrate bot the feasibility and safety of this approach. However, most subsequent relapses were again nodal in origin and were oligometastatic. A recent comparison of elective nodal radiotherapy and stereotactic body radiotherapy in patients with nodal recurrence. Distant failure was more common in patients treated with SBRT compared to ENRT. Further, those treated with SBRT tended to relapse more often in lymph nodes, likely reflecting the limited sensitivity of even these advanced imaging approaches to detect microscopic nodal disease.
Further, metastasis-free survival is improved with the use of ENRT, compared to SBRT, for patients with one positive lymph node. However, in those with more sites of disease, there was no difference between these approaches. Thus, those with a single nodal site of disease may represent a sufficiently early point in the disease trajectory to allow for true eradication of disseminated disease. Citing data from Princess Margaret Cancer Center, he emphasizes that PSMA-PET/CT may identify patients with oligorecurrent disease who may be suitable for curative-intent metastasis-directed therapy. Notably, more than one-fifth (22%0 may be rendered biochemically free of disease with metastasis-directed therapy alone. A PSMA-directed SBRT treatment approach for recurrent disease thus has both promising efficacies, but also acceptable toxicity.
Dr. Selek then discussed the recently published EMPIRE trial which randomized patients with biochemical recurrence following radical prostatectomy to receive salvage radiotherapy guided by conventional imaging of 18F-fluciclovine. The authors found that failure-free survival rates were significantly higher at three- and four years following index for those who received treatment informed by 18F-fluciclovine.
These data, combined with those from PMCC, raise the question of whether we need concomitant hormonal therapy. Recently published work from Dr. Kroze and colleagues showed that concurrent ADT improved biochemical recurrence-free survival. However, MDT alone can provide relatively high rates of ADT-free survival. Importantly, a recent analysis further showed that the use of concomitant ADT should exceed 12 months for maximal benefit. Additionally, Dr. Selek highlighted data from OLIGOPELVIS-GETUG-P07 which examined combined high-dose salvage radiotherapy with hormone therapy in patients with oligorecurrent pelvic nodal disease, finding that 2- and 3-year progression-free survival rates were 81% and 58%, respectively. A number of ongoing trials will assess the role of PSMA-PET/CT imaging to inform salvage radiotherapy treatment strategies. However, as of today, he suggested that we do not know which patients are best suited for these treatment strategies. However, considering both the potential for stage migration to inflate apparent outcome benefits and the outstanding questions to be addressed, the question of who and how patients may benefit from these treatment approaches is currently unclear.
Presented by: Prof. Uğur Selek, MD, Radiation Oncology, Turkey
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2021 Société Internationale D’Urologie (SIU) Hybrid Annual Meeting, Wed, Nov 10 – Sun, Nov 14, 2021.