(UroToday.com) The Société Internationale D’Urologie (SIU) 2021 annual meeting included a master class on advanced kidney cancer with a presentation by Dr. Kevin Lu discussing nephrectomy as part of the sequencing options in metastatic renal cell carcinoma (RCC) with the primary in place. Dr. Lu notes that we are in a rapidly evolving treatment landscape for metastatic RCC, highlighted by new drugs, drug combinations, drug sequences, vaccinations, improving local management, and precision therapy. As follows is a timeline of the current therapeutics available for advanced RCC:
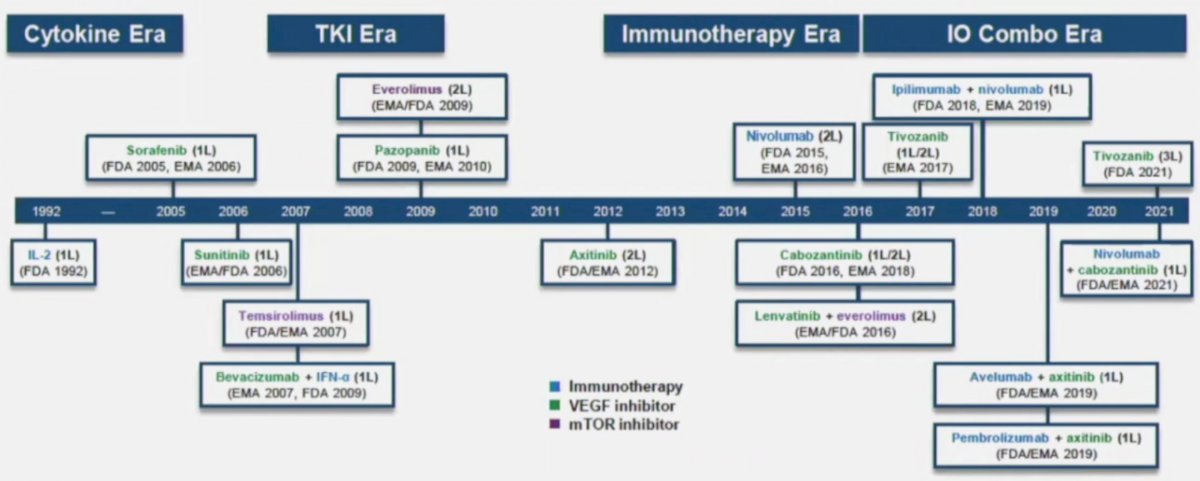
However, with more drugs, more drug classes, and the prospects of more to come, there are still unresolved questions and unmet needs. Dr. Lu notes that RCC behaves perhaps differently than other cancers in that some tumors are aggressive versus those that are indolent. Additionally, some RCCs recur frequently, some grow and metastasize quickly, while others grow slowly. Ultimately, the current information (clinical and pathologic data) is inadequate to differentiate between these entities. For all genitourinary malignancies, in particular RCC, it is important to assess:
- Clinical parameters: symptoms and lab results
- Pathologic parameters: molecular subtype, variant histology
- ‘Omics’ data: genomic profile of the tumor
- Nomograms: if available
Dr. Lu emphasized that in the contemporary IO-TKI trials, the majority of patients entered the trial after a prior radical nephrectomy, including 82% in the CheckMate 214 trial,1 83% in the KEYNOTE-426 trial,2 69% in the CheckMate-9ER trial,3 and 74% in the CLEAR trial,4 Furthermore, the advantage or role of cytoreductive nephrectomy is not particularly clear. In the cytokine therapy era, the SWOG trial had a survival advantage of 3 months, whereas the EORTC trial had a benefit of 10 months, with an aggregate survival advantage among these two trials of 5.8 months. In the targeted therapy era, Choueiri et al.5 reported a median OS of 19.8 months for patients undergoing cytoreductive nephrectomy versus 9.4 months for those not undergoing surgery (HR 0.44, 95% CI 0.32-0.59). Heng et al.6 reported a median OS of patients with cytoreductive nephrectomy versus without cytoreductive nephrectomy of 20.6 versus 9.5 months (p<0.0001), respectively. When adjusted for IMDC criteria to correct for imbalances, the HR of death was 0.60 (95% CI 0.52-0.69):

Finally, Hanna et al.7 noted that in the NCDB, the median OS of cytoreductive nephrectomy versus non-cytoreductive nephrectomy patients was 17.1 (95% CI, 16.3 to 18.0 months) versus 7.7 months (95% CI, 7.4 to 7.9 months; p < 0.001). However, for all three of these studies, the caveat is that they are all retrospective with inherent selection bias.
With regards to cytoreductive nephrectomy in patients treated with targeted therapy or immune checkpoint inhibitors, Dr. Lu highlighted work presented at the 2020 GU ASCO meeting by Dr. Bakouny et al. that showed cytoreductive nephrectomy was associated with improved overall survival in patients treated with either first-line targeted therapy (HR 0.48, 95% CI 0.45-0.52) or first-line immune checkpoint inhibitors (HR 0.44, 95% CI 0.30-0.64):

But as we know from CARMENA and SURTIME, not everyone will benefit from cytoreductive nephrectomy. In CARMENA,8 450 patients who were suitable candidates for nephrectomy were randomly assigned, in a 1:1 ratio, to undergo nephrectomy and then receive sunitinib or to receive sunitinib alone. Randomization was stratified according to prognostic risk (intermediate or poor) in the Memorial Sloan Kettering Cancer Center prognostic model, and the primary endpoint was overall survival. At the planned interim analysis, the median follow-up was 50.9 months, and the results in the sunitinib-alone group were non-inferior to those in the nephrectomy-sunitinib group with regard to overall survival (HR for death 0.89, 95% CI 0.71-1.10; upper boundary of the 95% confidence interval for noninferiority, ≤1.20). The median overall survival was 18.4 months in the sunitinib-alone group and 13.9 months in the nephrectomy-sunitinib group. The investigators concluded that cytoreductive nephrectomy should no longer be part of standard of care for patients with metastatic RCC requiring medical treatment.
The SURTIME trial assessed immediate surgery or surgery after sunitinib in treating patients with metastatic RCC.9 This study examined whether a period of sunitinib therapy before cytoreductive nephrectomy improves outcome compared with immediate cytoreductive nephrectomy followed by sunitinib. With an estimated sample size need of 458 patients to determine a difference in PFS, unfortunately, the study closed after 5.7 years with 99 patients accrued. The 28-week progression-free rate was 42% in the immediate cytoreductive nephrectomy arm (n = 50) and 43% in the deferred cytoreductive nephrectomy arm (n = 49) (p = 0.61). The intention-to-treat OS HR of deferred vs immediate cytoreductive nephrectomy was 0.57 (95% CI, 0.34-0.95; p = 0.03), with a median OS of 32.4 months (95% CI, 14.5-65.3 months) in the deferred CN arm and 15.0 months (95% CI, 9.3-29.5 months) in the immediate CN arm.
According to Dr. Lu, the place for nephrectomy in the trajectory of metastatic RCC for patients with the primary in situ is for patients that need symptom palliation, those with a response to initial systemic therapy, and those with low metastatic disease burden. The EAU guidelines have excellent recommendations for guiding clinicians when making these decisions as highlighted in the following:

There are several phase 3 ongoing trials assessing the role of cytoreductive nephrectomy and SBRT in the contemporary era of therapy. The NORDIC-SUN (NCT03977571) trial is an open-label phase 3 randomized trial of deferred cytoreductive nephrectomy in synchronous metastatic RCC receiving checkpoint inhibitors. Approximately 400 patients will be randomized to surgery after induction therapy (nivolumab + ipilimumab) followed by maintenance nivolumab versus induction therapy (nivolumab + ipilimumab) followed by maintenance nivolumab (no surgery). The primary outcome for this trial is overall survival over a minimum of three years of follow-up. The PROBE trial (SWOG – under development) will include patients with metastatic RCC treated with an immunotherapy-based regimen who had stable disease/partial response/complete response who will then be randomized 1:1 to cytoreductive nephrectomy versus a continued immunotherapy-based regimen. Third, the CYTOSHRINK (NCT04090710) trial is randomizing 78 patients to nivolumab + ipilimumab followed by SBRT to the renal lesion followed by additional nivolumab + ipilimumab followed by maintenance nivolumab versus nivolumab + ipilimumab followed by maintenance nivolumab. With immunotherapy combinations as the first-line therapy, Dr. Lu suggests the following scenarios for the future role of nephrectomy in metastatic RCC:

Dr. Lu concluded his presentation with the following take-home messages:
- Nephrectomy should rarely be performed in patients with poor-risk disease and those with rapidly progressive disease or high tumor burden who need systemic therapy
- Upfront nephrectomy may be considered for those patients with favorable/intermediate-risk disease who are candidates for active surveillance, patients that are candidates for oligometastasectomy, and those with symptomatic renal masses
- Deferred nephrectomy should be considered in patients with a strong response to systemic therapy
Presented by: Kevin Lu, MD, Division of Urology, Department of Surgery & Clinical Competences Center, Department of Medical Education, Taichung Veterans General Hospital, Taichung City, Taiwan
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 Société Internationale D’Urologie (SIU) Hybrid Annual Meeting, Wed, Nov 10 – Sun, Nov 14, 2021.
References:
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carinoma. N Engl J Med 2018;378(14):1277-1290.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1116-1127.
- Choueiri TK, Powles T, Burotto M, et al. Nivolumab plus cabozantinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2021 Mar 4;384(9):829-841.
- Motzer R, Alekseev B, Rha SY, et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N Engl J Med. 2021 Apr 8;384(14):1289-1300.
- Choueiri TK, Xie W, Kollmannsberger C, et al. The impact of cytoreductive nephrectomy on survival of patients with metastatic renal cell carcinoma receiving vascular endothelial growth factor targeted therapy. J Urol. 2011 Jan;185(1):60-66.
- Heng DYC, Wells JC, Rini BI, et al. Cytoreductive nephrectomy in patients with synchronous metastases from renal cell carcinoma: Results from the International Metastatic Renal Cell Carcinoma Database Consortium. Eur Urol. 2014 Oct;66(4):704-710.
- Hanna N, Sun M, Meyer CP, et al. Survival analyses of patients with metastatic renal cancer treated with targeted therapy with or without cytoreductive nephrectomy: A National Cancer Data Base Study. J Clin Oncol. 2016 Sep 20;34(27);3267-3275.
- Mejean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal cell carcinoma. N Engl J Med 2018 Aug 2;379(5):417-427.
- Bex A, Mulders P, Jewett M, et al. Comparison of immediate vs deferred cytoreductive nephrectomy in patients with synchronous metastatic renal cell carcinoma receiving sunitinib: The SURTIME Randomized Clinical Trial. JAMA Oncol 2019 Feb 1;5(2):164-170.