He focused his presentation on unpublished data and that which he felt had the greatest therapeutic implications. Thus, he began with a discussion of the AR regulation of PSMA. He began by highlighting that PSMA is inversely regulated by the androgen receptor. Thus, anti-androgen therapies have dramatic effects on PSMA expression. However, the kinetics is “a bit unusual” in that it takes about 2 weeks to see PSMA reach peak levels. At that time, there is a 5 to 10 fold upregulation of PSMA.
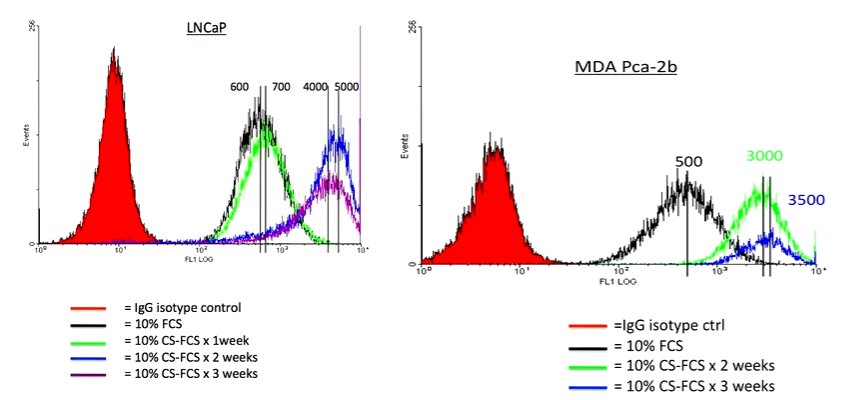
This is relevant as there are therapeutically targeted efforts to upregulate PSMA. Thus, this understanding of its biology is critical to inform interventions as well as imaging studies. This may further help to reconcile somewhat disparate results of existing imaging studies. Further, when considering imaging applications, there may be two conflicting phenomena: the anti-androgen therapy may serve to upregulate expression of PSMA while at the same time exerting an anti-tumor effect. Thus, the overall effect on PSMA expression may not be entirely predictable.
Further, Dr. Bander noted that the effects of castration on PSMA expression are, interestingly, not limited to hormone-responsive cells. Thus, in CWR22rv1 cell lines that exhibit castration resistance, castration still prompts an increase in PSMA expression. Thus, the biology of PSMA remains important even as we transition to castration-resistant disease.
Dr. Bander then moved to discuss internalization of antibodies and ligands targeting PSMA. Dating back to 1998, Dr. Bander and colleagues demonstrated for the first time that PSMA internalized, through a novel cytoplastic tail MXXXL motif. He noted that, based on confocal video microscopy, PSMA is rapidly and efficiently internalized and localizes to lysosomes, taking up a location adjacent to the nucleus. This is a critical feature in the development of radiopharmaceuticals as it puts a PSMA-antibody attached payload in proximity to the nucleus to facilitate DNA damage. Dr. Bander further pointed out that the residence time of the agent is also important in therapeutic radiopharmaceuticals.
In both castration sensitive and castration resistant cell models, he noted that there are differences in internalization between different agents, with ongoing internalization over time with J591 antibody, in contrast to the plateau exhibited with PSMA-617 which occurred around 2 hours and was much lower than see with the antibody.
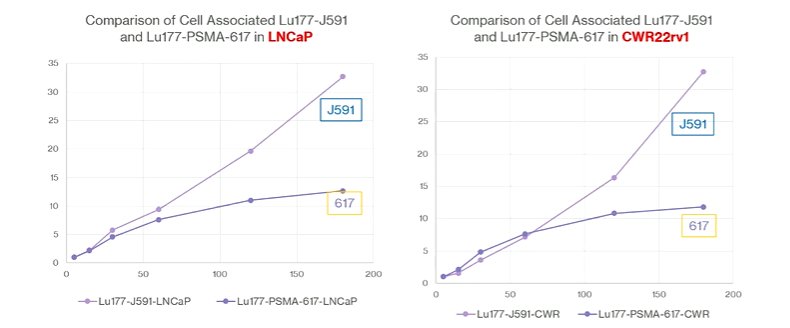
He postulated, based on these data, that PSMA-617 was being externalized simultaneously, preventing high-level accumulation. In doing the reverse experiment, they showed that there was dramatically faster washout of PSMA-617 than J591 regardless of cell line used. Interestingly, however, in the LNCaP cell lines, it reaccumulated to some degree after a trough around 4 hours whereas this was not observed in the castration-resistant CWR22rv1 cell line. This represents reuptake in the castration-sensitive cells that is not present in the castration-resistant line. He postulates that this is due to an increased number of PSMA molecules on the castration-sensitive cells to allow for reuptake.
He noted that, as a result of these findings, radioligands have relatively short cellular retention time, in a way that limits the dose deliverable to the tumor. While both the J591 and PSMA-617 are internalized by an endocytic pathway in which the endosomal pH drops to approximately 5.5, PSMA-617 is increasingly unbound from PSMA at lower pHs. When the endosome recycles to the cellular membrane, the ligand is expelled. In contrast, the antibody does not dissociate (until pH is lowered to 2.5), thus it is retained and is not externalized. Prior work from Germany has also shown that 60-75% of the ligand is lost via externalization by one hour following administration. Dimerization provided only marginal benefit.
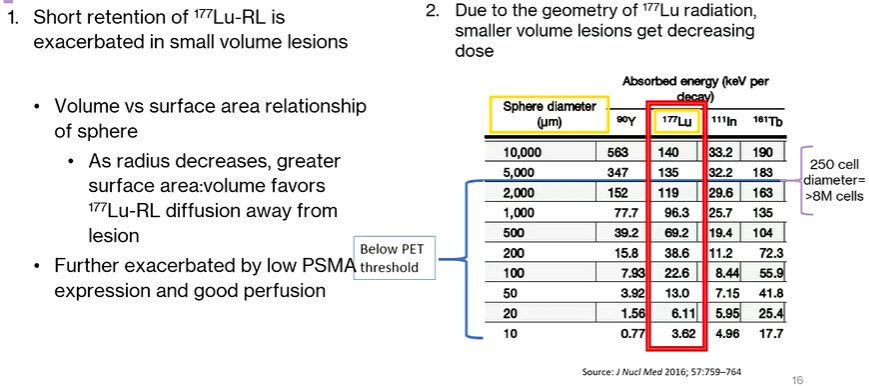
Moving this to clinical applications, Dr. Bander discussed the potential consequences of limited radioligand retention. These include a number of clinical observations that patients have developed new sites of disease, based on a postulation that 177Lu is less effective in treating microscopic deposits or marrow disease. Thus, progression following 177Lu is characterized here by the progression of previously unseen lesions as new foci of disease, rather than progression of existing disease with growth through therapy.
This prompts Dr. Bander’s hypothesis that 177Lu radioligand therapy under-treated small volumes of disease, for two reasons. First, the short retention of 177Lu-PSMA is exacerbated in small volume lesions. Second, due to radiotherapy geometry, there is a decreasing dosage administered to smaller lesions.
Understanding of the PSMA biology and macrostructure, Dr. Bander noted that small molecular ligands and the J591 antibody do not compete for binding. Instead, they actually bind non-competitively and additively. In fact, the combination of the two agents actually results in an increase in the update of the radioligand: adding unbound antibody increases the uptake of the ligand within the cell by 2-3 fold and adding bound antibody increased this even further.
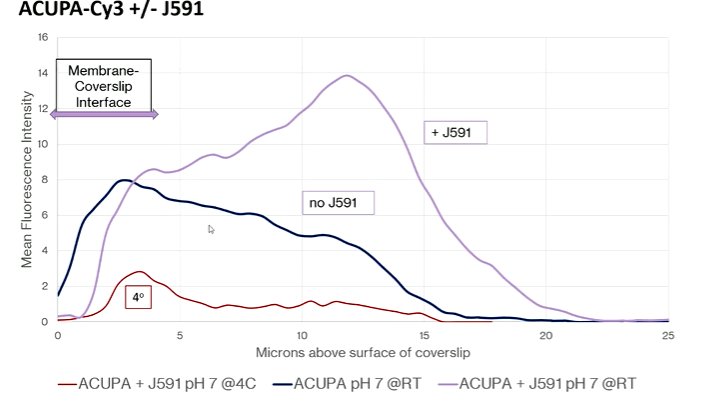
Further confocal microscopy work demonstrated that the addition of the J591 antibody resulted in an ongoing rise in the amount of intracellular ligand that is not seen in its absence. The authors observed that the antibody was driving the ligand to a lysosome from the recycling endosome, thus keeping it within the cell. Further, in in vivo models, there is increasing radioactivity accumulation within tumors seen when J591 is concurrently administered.
Interestingly, this effect is seen despite the fact that the small molecule ligand and the J591 antibody have non-overlapping biodistributions. As a result, there is non-overlapping toxicity which may make combinations of the two more tolerable.
Thus, the data suggests the potential, in patients, to provide a synergistic uptake with this dual-targeted approach. First assessing this in animal models, his group demonstrated that the measured uptake from combined administration exceeded the predicted uptake from the administration of the two. Thus, co-administration of the antibody resulted in an increased dose delivery to the tumor. This was validated in autopsy studies. Across multiple animal models and cell lines, the authors observed the same results, with a synergistic effect of co-administration.
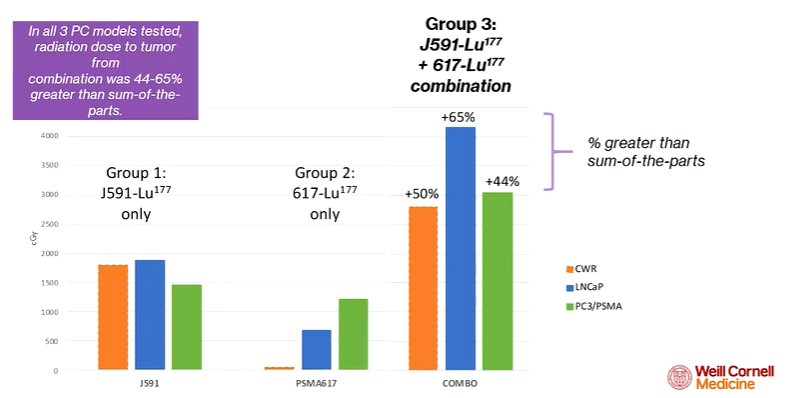
Further, he noted that the antibody uptake did not appear to depend on the PSMA expression of the cell line. In contrast, uptake of the ligand was highly dependent on the amount of PSMA on the cell membrane.
In terms of therapeutic significance, in vivo studies provide a better therapeutic result from the combination.
Thus, in conclusion, he noted that both small molecular ligands and J591 antibody radionuclides have favorable activity. However, co-administration appears to have additional benefit. In pre-clinical data, this shows promising synergistic activity. However, a trial exploring this is underway to better assess its clinical implications.
Written by: Christopher J.D. Wallis, University of Toronto Twitter: @WallisCJD during the 2022 European Society for Medical Oncology (ESMO) Annual Congress, 9-13 September 2022.