(UroToday.com) The 2021 European Society of Medical Oncology’s (EMSO) annual congress included a proffered paper session in non-prostate genitourinary tumors, as well as a discussant presentation by Dr. Christian Kollmannsberger discussing decreasing morbidity for the treatment of stage II seminoma. The abstract discussed was “Single-dose carboplatin followed by involved-node radiotherapy as a curative treatment for seminoma stage IIA/B: Efficacy results from the international multicenter phase II trial SAKK 01/10” by Dr. Alexandros Papachristofilou. Indeed, the price for a cure in testicular cancer is often at the cost of long-term morbidity/mortality from treatment. The SAKK 01/10 trial attempted to decrease treatment burden while maintaining cancer efficacy. This trial is a multicenter, single-arm, phase II study in patients with clinical stage IIA/B seminoma (de novo or relapse on active surveillance). Treatment consisted of 1 cycle carboplatin AUC7 followed by involved-node radiotherapy (IIA: 30 Gy; IIB: 36 Gy) with a primary endpoint of 3-year PFS:

There were 120 patients enrolled, including 116 evaluable patients with a minimum follow-up from inclusion of the last patient is 3 years, with a median follow-up time of 4.5 years (range: 0.8 years - 8.1 years). Importantly, the median planned target volume was ~25% of a typical, standard of care dog-leg planned target volume, thus a 75% median decrease in irradiated volume.
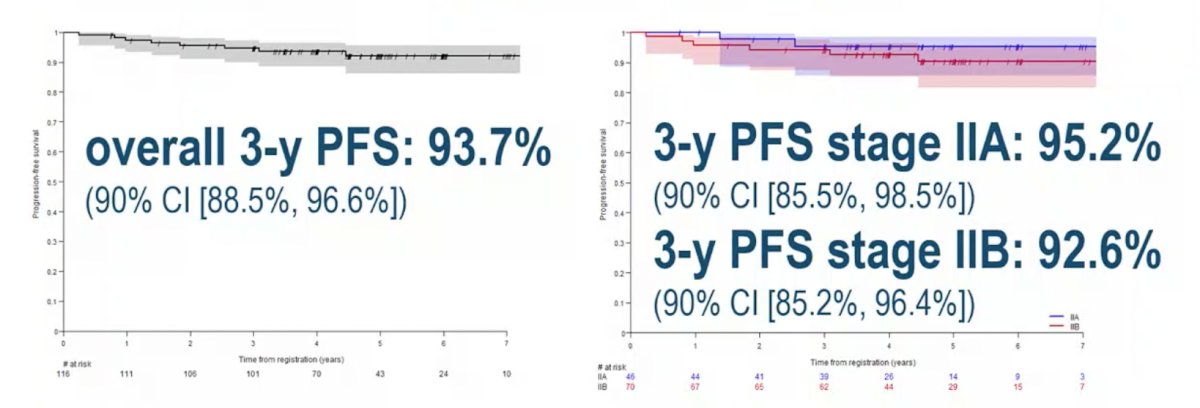
The 3-year PFS rate is 93.7% (90% CI 88.5%, 96.6%), with a 3-year PFS rate of 95.2% (90% CI 85.5%, 98.5%) for IIA patients and 92.6% (90% CI) for IIB patients:
Dr. Kollmannsberger notes that there have been several attempts to reduce the treatment burden in metastatic seminoma:
- Retrospective data collection of 216 patients from 3 UK centers (2008-2018) treated with 3-4 cycles of carboplatin AUC 10
- A phase II study of 48 patients of 3-4 cycles of carboplatin AUC 10 guided by PET
- SEMITEP trial (n=102): de-escalating chemotherapy based on early PET findings
All of these trials claimed positive results and a potential new standard of care, but none of these trials are generally accepted as the standard of care in 2021.
When attempting to change the standard of care there are several ‘Musts’ and ‘Must-nots’ that need to be considered:
- Musts:
- Very high confidence that de-escalation efforts will not diminish cure rate with initial management
- Address high-impact questions with meaningful effect size, incorporate high-quality, low complexity, and reliable, safe trial methodologies and measure high fidelity endpoints
- Enroll a sufficiently large number of patients and have a strong clinical and biological plausibility
- Results must be meaningful and resulting treatment change must be easily actionable at a provider, patient, and community level
- Treatment should be simple and easily reproducible in a variety of care settings
- Must-Nots: no preconceived assumptions regarding the benignity of the intervention in terms of toxicity or efficacy if the intervention could conceivably threaten the current cure rate
With regards to the SAKK 01/10 trial, Dr. Kollmannsberger has several concerns and questions regarding the trial design and conduct, including (i) recruitment was from 2012-2018 with only 1 patient per center per year; was there patient selection bias? (ii) how was confirmation diagnosed (up to 30% of stage IIA patients are not testicular cancer)? (iii) the number and size of lymph nodes in IIB patients, how many were < 3 cm >? (iv) formally, the results are not statistically significant based on the pre-specified analysis plan, (v) RT plans – how many were actually centrally reviewed? (vi) how many of the relapses were in the original dogleg? (vii) follow-up is too short to conclusively assess outcomes and long-term toxicity, (viii) was toxicity formally tested? (ix) the treatment plan adds more complexity and even more risk for variation in care.
It is important for context to understand the adverse health outcomes in testicular cancer survivors that have been treated with chemotherapy. Fung et al. [1] assessed quality of life among 952 patients receiving either EPx4 or BEPx3/BEPx4 with a median time since chemotherapy of 4.3 years. None, one to two, three to four, or five or more adverse health outcomes were reported by 20.4%, 42.0%, 25.1%, and 12.5% of testicular cancer survivors, respectively. The median number after EPX4 or BEPX3 was 2 (range: 0 to 9 and 0 to 11, respectively; p > .05) and two (range: 0 to 10) after BEPX4. Dr. Kollmannsberger notes that the majority of patients describe their quality of life after BEPx3 as excellent/very good:

Carboplatin is clearly inferior in metastatic seminoma with very modest activity as adjuvant therapy in clinical stage I therapy. Additionally, based on data presented at GU ASCO 2021, Dr. Kollmannsberger has concerns with regards to the combination of chemotherapy and radiotherapy given that in the Norwegian Cancer Registry the combination increased second cancer incidence and non-second cancer-related mortality. Collectively, there has been a recent emphasis on improving testicular cancer outcomes, specifically related to the development of biomarkers, improvement in care delivery, and a focus on cancer survivorship.
Dr. Kollmannsberger concluded his presentation with the following take-home messages:
- For the SAKK 01/10 trial, therapeutic equivalence/non-inferiority has not been demonstrated
- There are questions remaining with regards to long-term efficacy and toxicity
- Much longer follow-up is needed to conclusively assess outcomes
- Carboplatin AUC7 plus involved nodal radiotherapy is not a standard of care
Presented by: Christian K. Kollmannsberger, MD, University of British Columbia, Vancouver, BC, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References:
- Fung C, Sesso HD, Williams AM, et al. Multi-institutional assessment of adverse health outcomes among North American Testicular Cancer Survivors after Modern Cisplatin-Based Chemotherapy. J Clin Oncol. 2017 Apr 10;35(11):1211-1222.