(UroToday.com) In this study, Dr. Karim Fizazi discussed the health-related quality of life (HRQoL) data from the VISION study of 177Lu-PSMA-617 (Lu-PSMA). While the results of the VISION study, published earlier this year, demonstrated a significant improvement in radiographic progression-free survival (rPFS) and overall survival (OS) for men with metastatic castration-resistant prostate cancer (mCRPC), this is the first report of the HRQoL data.1
The VISION study is a randomized Phase 3 clinical trial of men with mCRPC previously treated with at least one androgen receptor pathway inhibitor and one or two taxane chemotherapy regimens. Patients had to have a PSMA-positive lesion on Gallium-PSMA PET/CT – 87% of patients scanned met the imaging criteria for inclusion. Eligible participants were randomized to protocol-permitted standard-of-care (SOC) therapy versus SOC plus six cycles of Lu-PSMA.
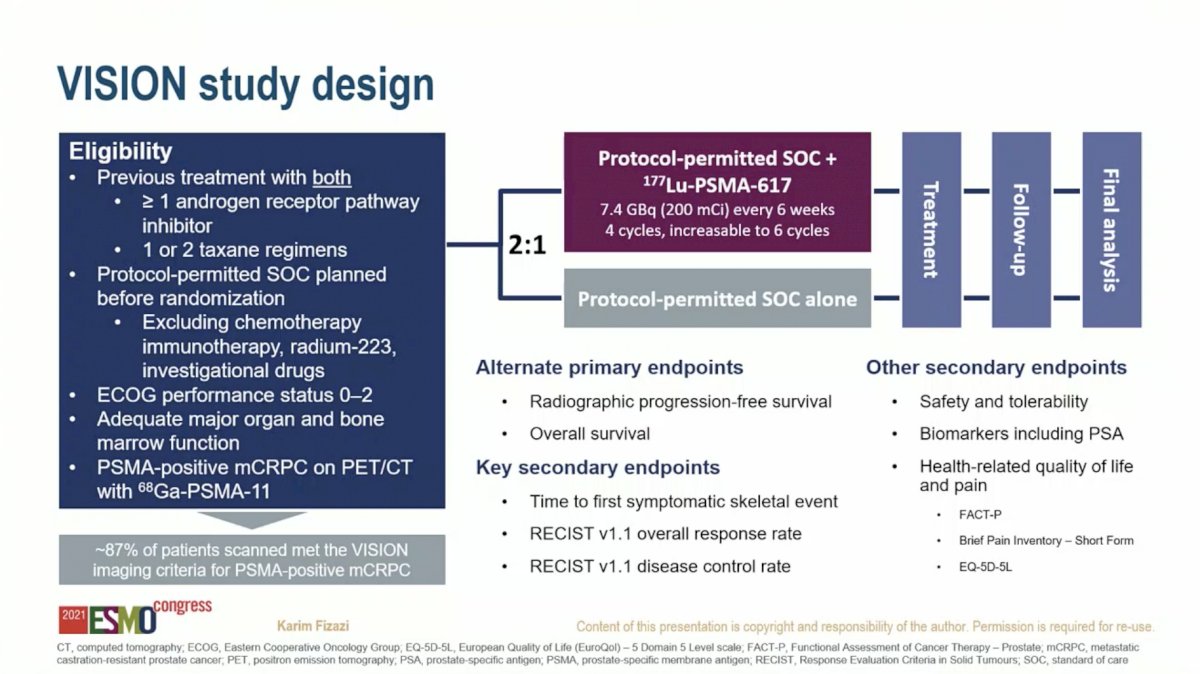
The first outcome reviewed by Dr. Fizazi was time to first skeletal event, a pre-specified key secondary endpoint. The addition of Lu-PSMA to SOC extended the median time to symptomatic skeletal event from 6.8 months to 11.5 months (HR 0.50, 95% CI 0.40-0.62; P < 0.001).

The two patient-reported outcomes evaluated in this study were the FACT-P score and the BPI-SF pain intensity score. Time to worsening for both of these metrics also favored Lu-PSMA with a delay in time to worsening of 7.3 months for the FACT-P score and 11.4 months for the BPI-SF pain intensity score.

An updated assessment of treatment-emergent adverse events (AEs) confirmed that the addition of Lu-PSMA was generally well-tolerated. The most common Grade 3-5 AE in patients treated on the Lu-PSMA arm was bone marrow suppression, which occurred in 23.4% of patients compared to 6.8% on the SOC arm. Dry mouth and nausea and vomiting were also common AEs in patients treated with Lu-PSMA (bother 39.3%), but both were largely limited to Grade 1-2 events.

Dr. Fizazi concluded that the combination of Lu-PSMA and SOC in patients with mCRPC previously treated with an androgen receptor pathway inhibitor and taxane chemotherapy extended OS, delayed rPFS, and was generally well-tolerated. Further, results presented herein support that Lu-PSMA delayed time to worsening in HRQol and pain, and delayed time to first symptomatic skeletal events.
Presented by: Karim Fizazi, MD, PhD, Medical Oncologist at Gustave Roussy and Professor in Oncology at the University of Paris-Saclay in Villejuif, France
Written by: Jacob Berchuck, MD, Genitourinary Medical Oncologist, Dana-Farber Cancer Institute (Twitter: @jberchuck) during the 2021 European Society for Medical Oncology (ESMO) Annual Congress 2021, Thursday, Sep 16, 2021 – Tuesday, Sep 21, 2021.
References: