In this study, clinical data were abstracted from 109 men with mCRPC who were treated with cabazitaxel in the Netherlands. Many patient characteristics were similar between the real-world cohort and the clinical trial cohort, including age, time on androgen deprivation therapy, alkaline phosphatase, median PSA, ECOG performance status, and opioid use.
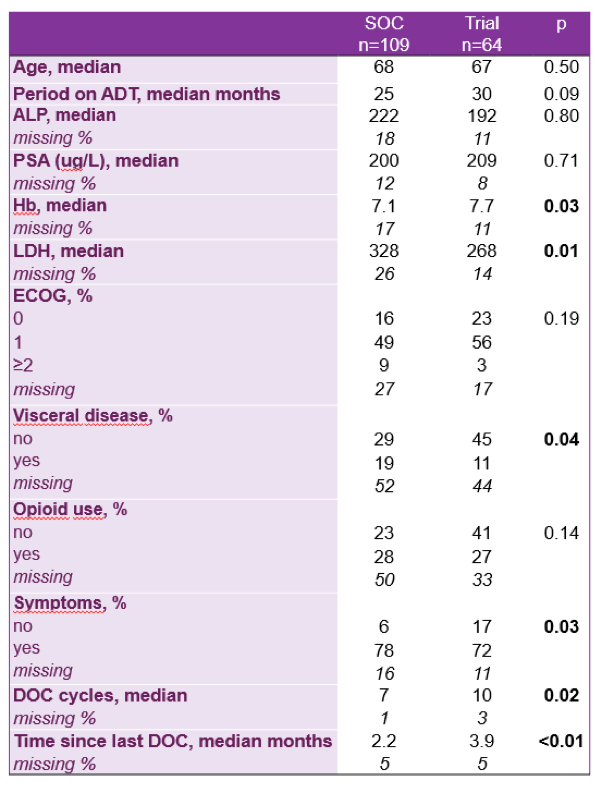
However, a few differences did exist. Those treated in outside of the clinical trial had a lower hemoglobin (7.1 vs 7.7), higher LDH (328 vs 268), more visceral disease (19% vs 11%), were symptomatic (78 vs 72%), had fewer cycles of docetaxel (7 vs 10), and had a shorter time since last docetaxel infusion (2.2 months vs 3.9 months). The median overall survival for non-trial patients was 9.6 months, which is similar to what Oudard et al reported as well. This is likely due to treating a sicker population of patients outside of clinical trials, as described by the differences in patient characteristics above.

Figure 1: Overall survival cabazitaxel directly post-docetaxel, SOC vs in trial
This study demonstrates that patients treated with cabazitaxel as part of the standard of care may not have the same overall survival as those who were recruited for the original TROPIC clinical trial. This is largely due to a greater number of negative prognostic factors in the real world cohort, as more patients had a visceral disease, higher LDH, and lower hemoglobin. Future studies should investigate whether any biomarker is able to help risk stratify and choose the best candidates for cabazitaxel after docetaxel failure in patients with mCRPC.
Presented by: Hans M. Westgeest, Medical Oncologist, Breda, the Netherlands
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany