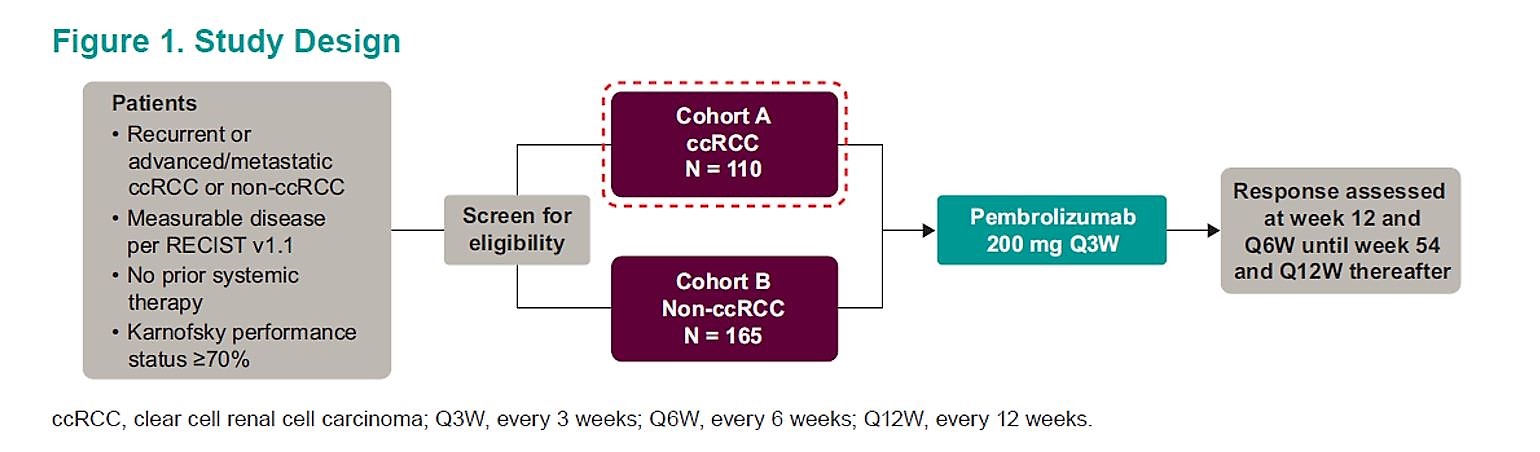
While there are several ongoing combination studies, not much is known about frontline single agent immune checkpoint blockade. The study design is shown below. In brief, patients with recurrent or advanced RCC were given single agent pembrolizumab 200 mg every 3 weeks. Patients were sorted by their tumor histology, with cohort A containing clear cell and cohort B containing a non-clear cell. We are reporting on cohort A at this time. The primary endpoint of this study was objective response rate.
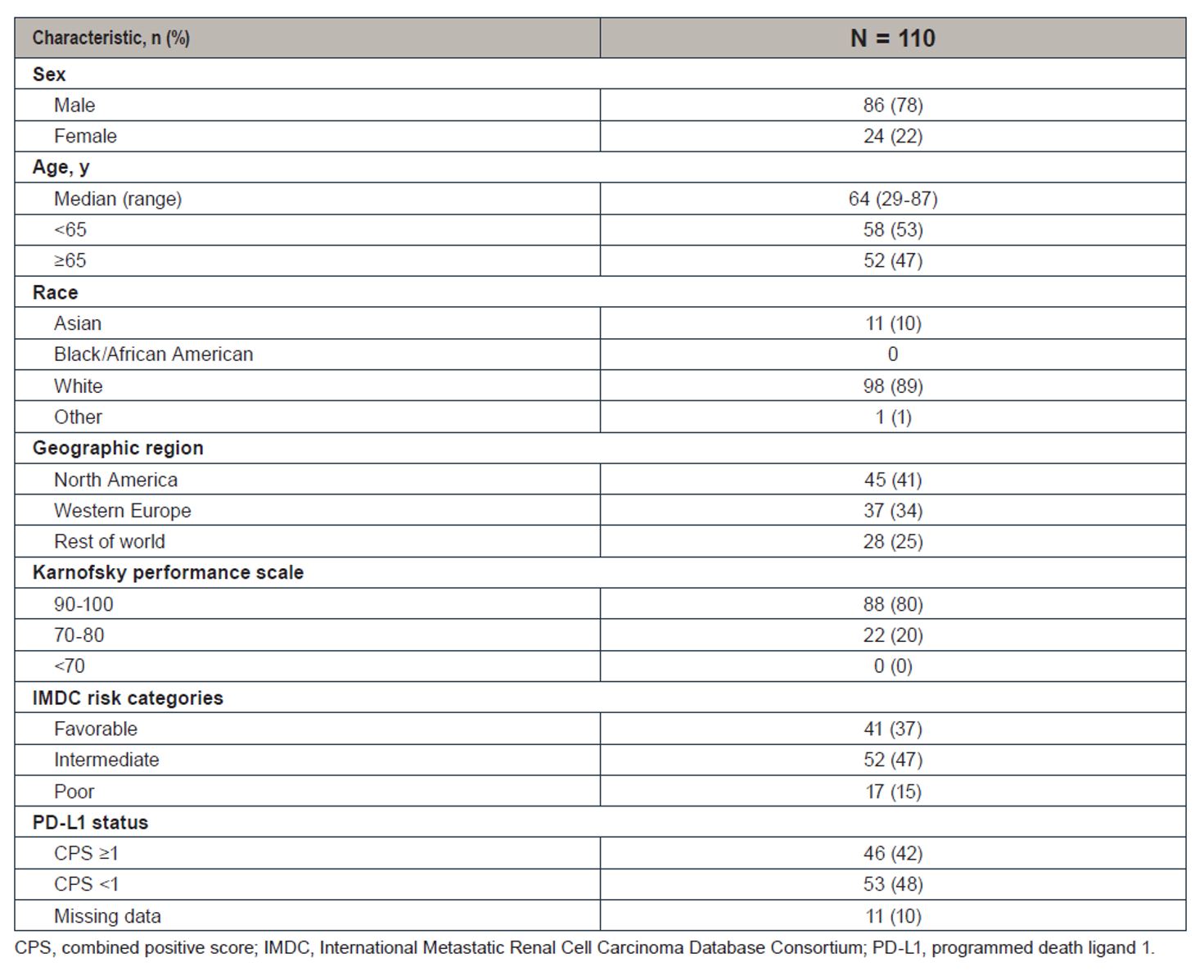
At the time of this assessment, more than half of the patients (45%) are still on treatment. In terms of baseline characteristics, most patients were IMDC favorable (37%) and intermediate (47%). 78% were men and 47% were older than 65. 89% were white and 10% were Asian. In terms of PD-L1 status and 42% had a combined positive score ≥1.
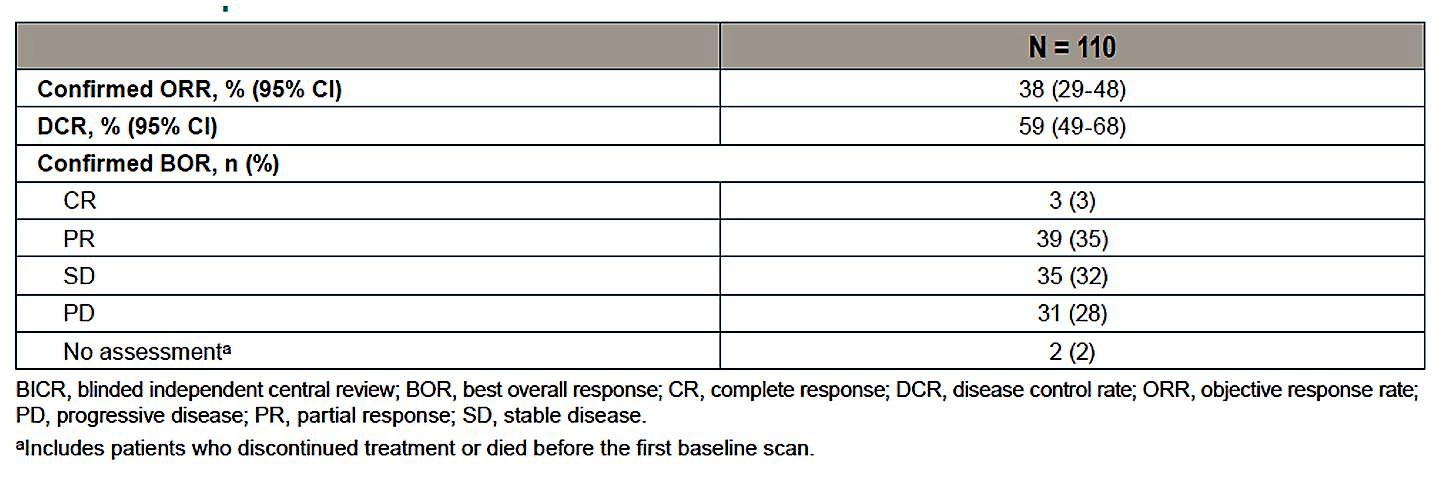
In terms of efficacy, the overall response rate was 38%, with most patients obtaining either a partial response (35%) or stable disease (32%). There were 3 complete responses (3%). Disease control rate was 59%.
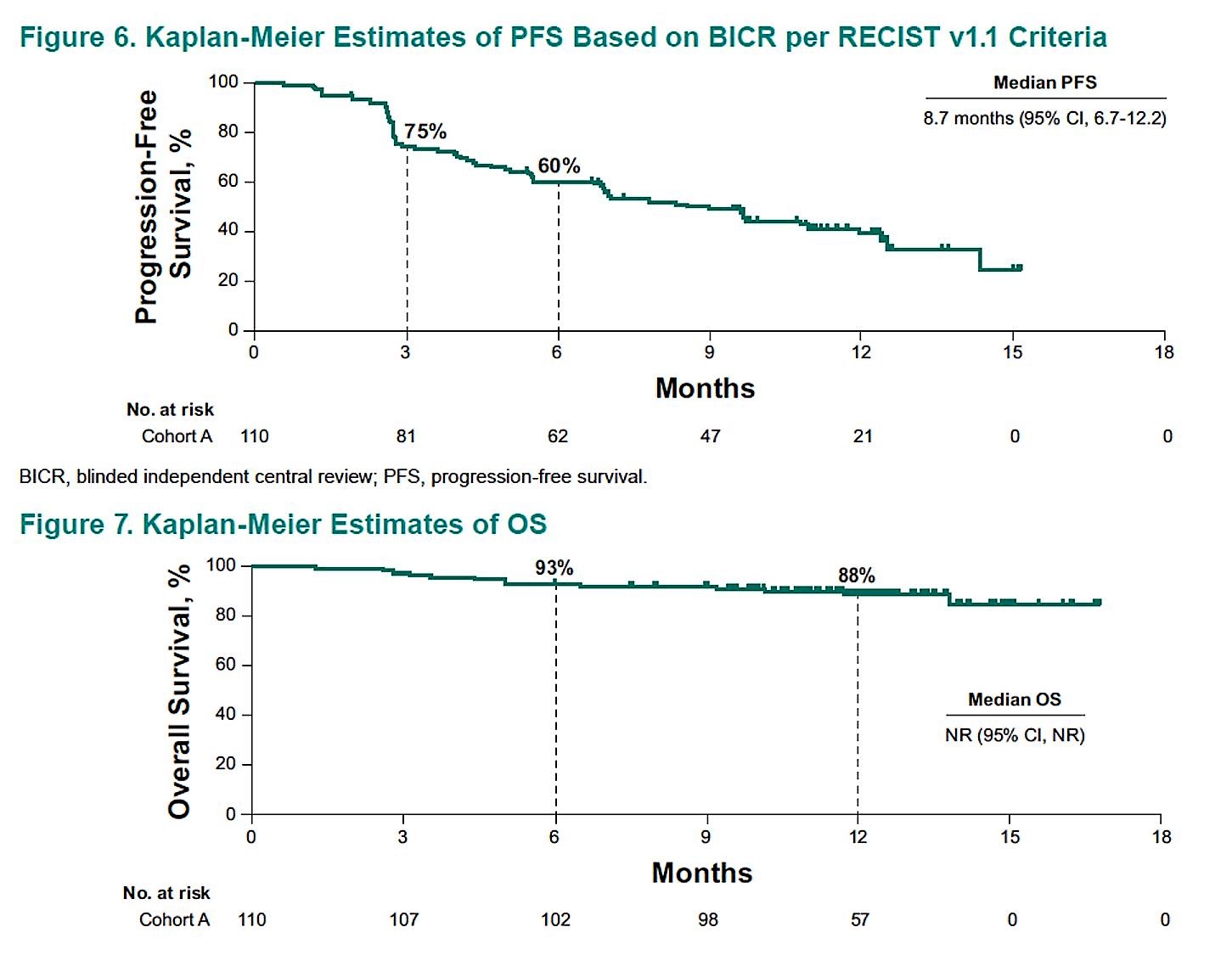
The median progression-free survival was 8.7 months and overall survival at 6 months was 93%. The median overall survival has not been reached.
In terms of response by IMDC risk, disease control rate was 66% in favorable risk and 55% in the intermediate/poor risk cohort. Confirmed ORR was higher in the intermediate/poor (42%) than favorable risk (32%).

Patients with sarcomatoid differentiation did exceptionally well with a confirmed objective response rate of 64% and disease control rate of 73%. Of note, this is a small cohort, with only 11 patients.
In terms of safety, almost all patients (80%) had a treatment-related adverse event, most commonly fatigue, pruritus, diarrhea, and rash. 22% of patients experienced a grade 3-5 treatment-related adverse event. The incidence of immune-related AEs appears similar in this study as other pembrolizumab studies.

This abstract demonstrates that single agent pembrolizumab has good disease control for patients with mRCC. The median progression-free survival of 8.7 months is similar to that of sunitinib (9.5 months) and pazopanib (8.4 months) and the overall survival of 92.4% at 6 months is similar to that of sunitinib in prior studies2,3. Not surprisingly, PFS with single-agent pembrolizumab is lower than the combination of ipilimumab+nivolumab (11.6 months)4. Longer follow up data may change this PFS and it will be interesting to see what overall survival is in this trial. Further studies are necessary to determine what the optimal sequence of therapy is for patients who are not candidates for ipi/nivo up but may be a candidate for single agent pembrolizumab or a TKI. Also, while intermediate/poor risk patients did not appear to receive benefit with ipi/nivo over sunitinib in CheckMate 2144, single agent pembrolizumab appears active in the good risk population as well. Lastly, for patients with sarcomatoid differentiation, immune checkpoint inhibition appears very effective in a number of studies, this one is no exception, and these patients should be considered for immunotherapy.
Presented By: Frede Donskov, MD, DMSci, Department of Oncology, Aarhus University Hospital, Aarhus, Denmark
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany
References:
1. Motzer RJ, Powles T, Atkins MB, et al. IMmotion151: A Randomized Phase III Study of Atezolizumab Plus Bevacizumab vs Sunitinib in Untreated Metastatic Renal Cell Carcinoma (mRCC). Journal of Clinical Oncology 2018;36:578-.
2. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus Interferon Alfa in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine 2007;356:115-24.
3. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus Sunitinib in Metastatic Renal-Cell Carcinoma. New England Journal of Medicine 2013;369:722-31.
4. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. New England Journal of Medicine 2018;378:1277-90.