This abstract describes the efficacy of frontline axitinib for the treatment of papillary RCC. The primary endpoint was 24-week progress free rate. Secondary objectives included safety, progression-free survival, overall survival, and best response rate.
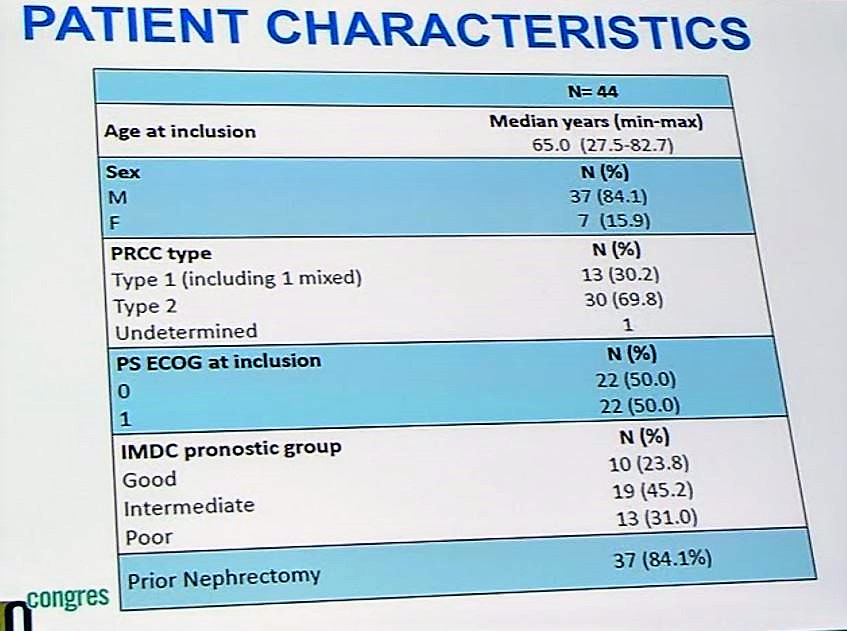
56 patients were enrolled and underwent a central pathology review. This is important as 5 patients were discovered to not have papillary RCC. The discussant notes that it is critical to have critical pathology review of papillary RCC cases because misclassification is not uncommon. Of the 50 remaining cases, 13 were Type 1 and 30 were Type 2, and ultimately 44 were given therapy.
Most patients were men (84%) and the median age was 75. All had a performance status of 0 or 1 and the majority had intermediate or poor IMDC prognosis. 84% of patients had prior nephrectomy.
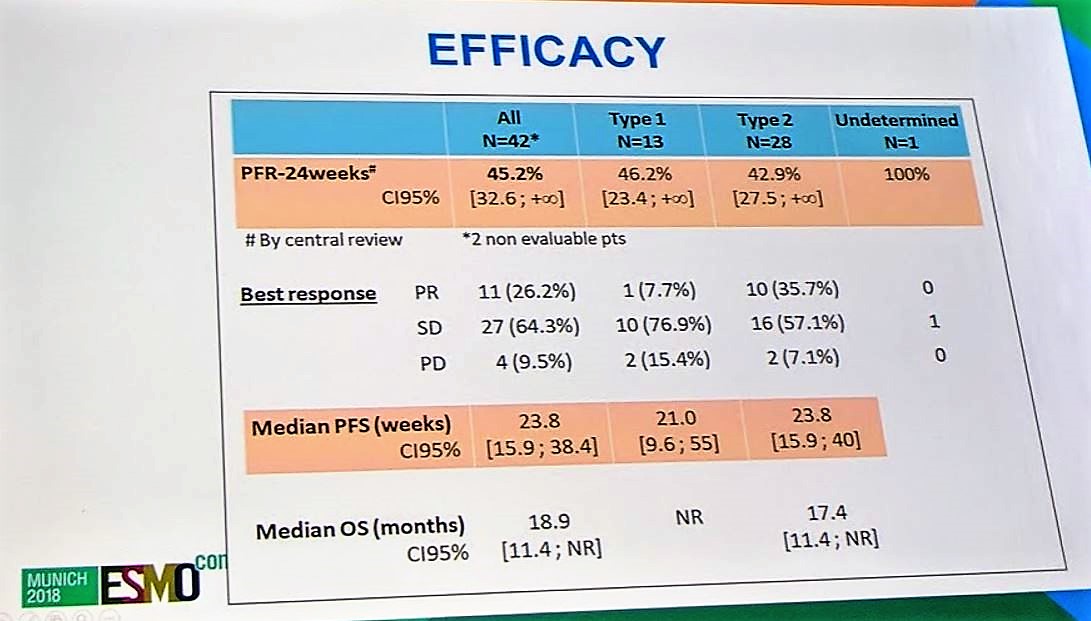
In terms of efficacy, 26% of patients had a partial response, 64% of patients had stable disease, and 9.5% of patients had progressive disease. Median PFS was 23.8 weeks for all patients and numerically similar for papillary type 1 and papillary type 2 (21.0 and 23.8 months). Median overall survival was 18.9 months for all patients and has not been reached for papillary type 1 patients.
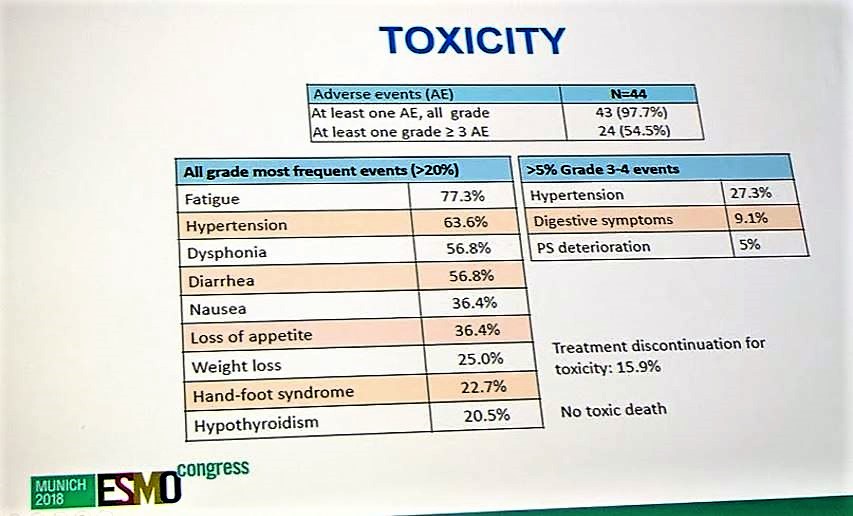
In terms of toxicity, 15.9% percent of patients had treatment discontinuation due to SAEs. There were no unexpected toxicities. The most common grade 3/4 SAEs were hypertension, GI symptoms, and deterioration in performance status.
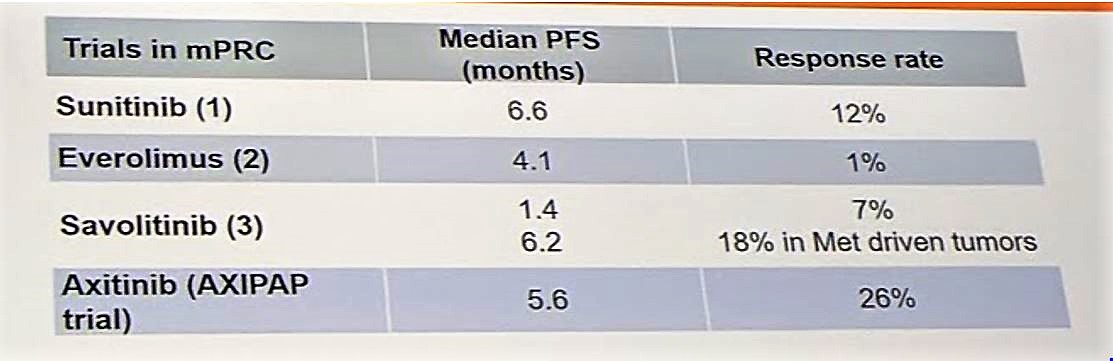
In this study, axitinib demonstrated substantial disease control for patients with papillary RCC and has a median PFS similar to other therapies for this indication.
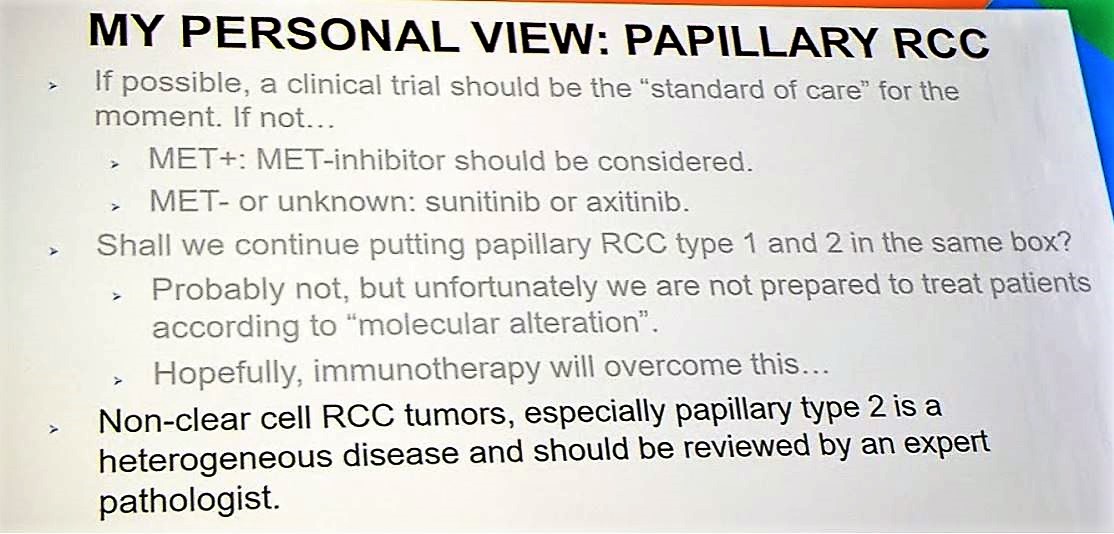
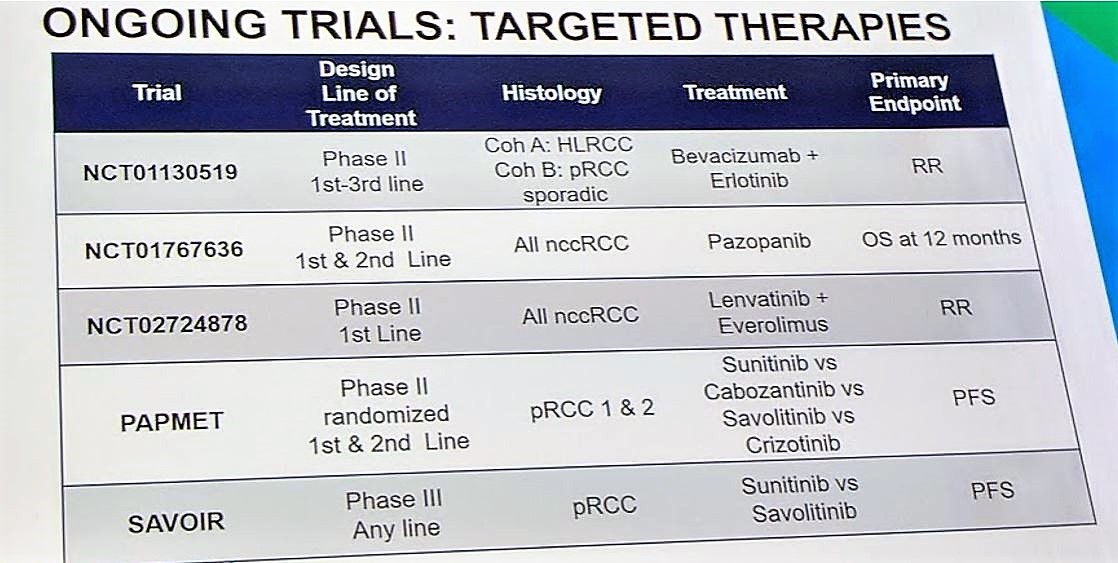
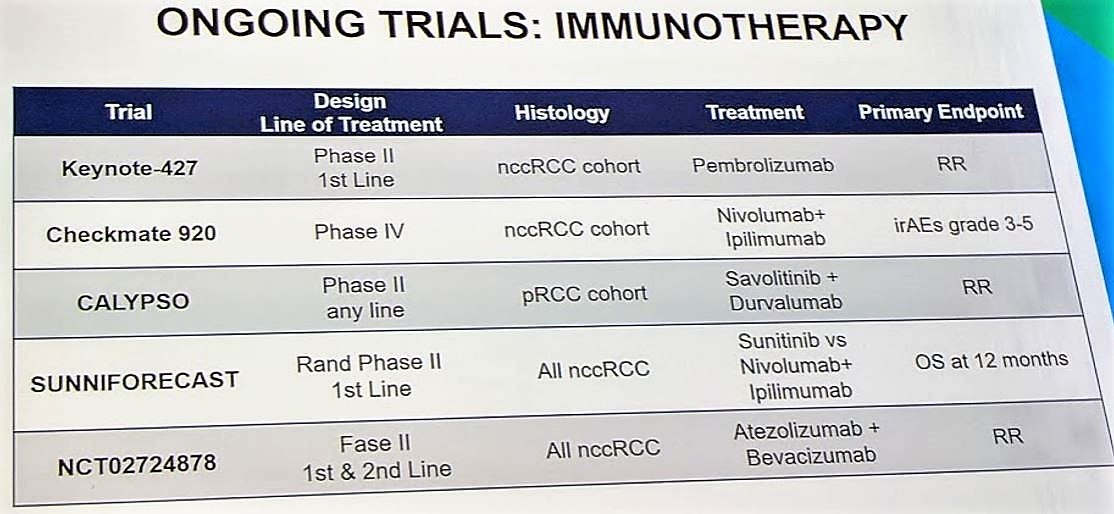
Given the rarity of papillary RCCs, it is important for patients with pRCC be enrolled in clinical trials so that we can develop strategies to best target this challenging disease. One such study is PAPMET, which is randomizing patients to one of four treatment arms: sunitinib, cabozantinib, crizotinib, and volitinib (NCT02761057). There are several targeted therapy studies and immunotherapy studies ongoing, which will be critical for developing the treatment paradigm for pRCC. Per the discussant, MET status should be determined for all patients with papillary RCC, and if MET positive, consideration should be given to MET inhibitors. If MET status is unknown or wildtype, then sunitinib and axitinib should be considered.
Presented By: Sylvie Negrier, MD, PhD, Centre Leon Berard, Lyon, France
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany
References:
1. Allory Y, Ouazana D, Boucher E, Thiounn N, Vieillefond A. Papillary renal cell carcinoma. Virchows Archiv 2003;442:336-42.
2. Armstrong AJ, Halabi S, Eisen T, et al. Everolimus versus sunitinib for patients with metastatic non-clear cell renal cell carcinoma (ASPEN): a multicentre, open-label, randomised phase 2 trial. The Lancet Oncology 2016;17:378-88.
3. Choueiri TK, Plimack ER, Arkenau H-T, et al. A single-arm biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer (PRCC). American Society of Clinical Oncology; 2017.H-TChoueiri TK, Plimack ER, Arkenau H-T, et al. A single-arm biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer (PRCC). American Society of Clinical Oncology; 2017.savolitinibChoueiri TK, Plimack ER, Arkenau H-T, et al. A single-arm biomarker-based phase II trial of savolitinib in patients with advanced papillary renal cell cancer (PRCC). American Society of Clinical Oncology; 2017.