Based on several other pre-clinical data as well as clinical evidence of efficacy in other tumor types, a randomized phase 2 study was conducted for patients with platinum-refractory advanced or metastatic UC2.In this study, ramucirumab plus docetaxel significantly improved median progression-free survival compared with docetaxel alone (5.4 months vs 2.8 months; HR 0.39; p=0·0002)2. However, there was no significant difference in overall survival in this study during the interim analysis – the authors postulated that the study was not powered to detect a difference in overall survival. Thus, this phase III study was conducted to assess if ramucirumab plus docetaxel is superior to docetaxel alone in the second line in terms of overall survival.
Patients with locally advanced or metastatic UC who had progressed on one or more platinum based regimens were randomly assigned to ramucirumab + docetaxel or placebo + docetaxel. Docetaxel was given at a dose of 75 mg/m2 every 3 weeks. Patients were stratified by geography, performance status, and the presence of visceral metastases.

In this updated report, the progression free survival data still shows a positive result, consistent with the earlier report. The ramucirumab + docetaxel arm had a PFS of 4.07 months compared to 2.76 months in the control arm of docetaxel, with an HR of 0.69 (95%CI 0.573-0.845, p=0.0002).

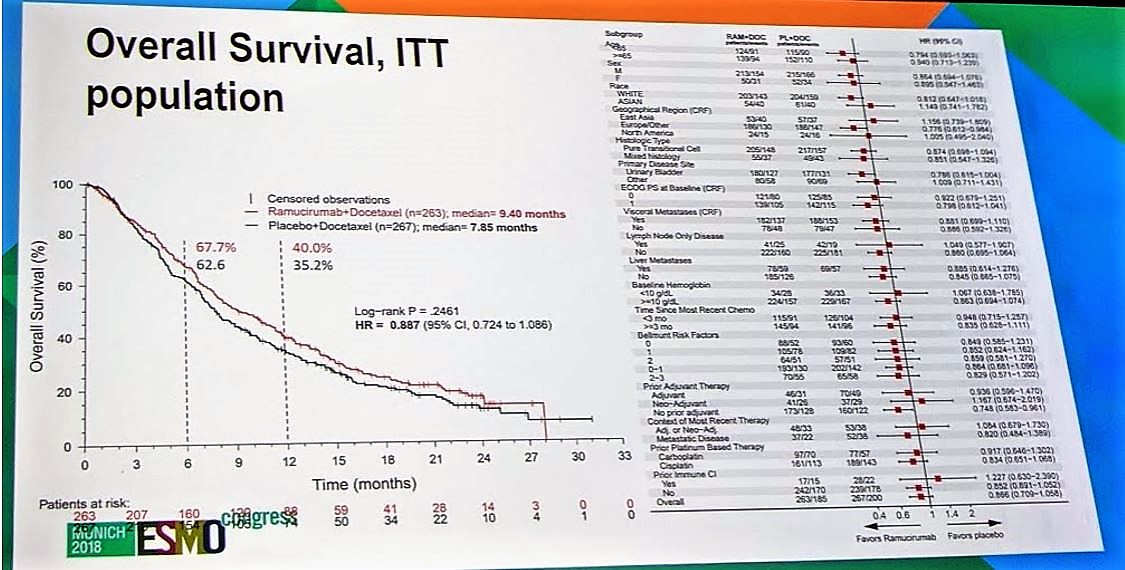
Overall survival was a secondary endpoint. Unfortunately, ramucirumab + docetaxel did not demonstrate an OS benefit compared to single-agent docetaxel (9.4 months vs 7.85 months, p=0.2461). This finding was consistent throughout all subgroups.
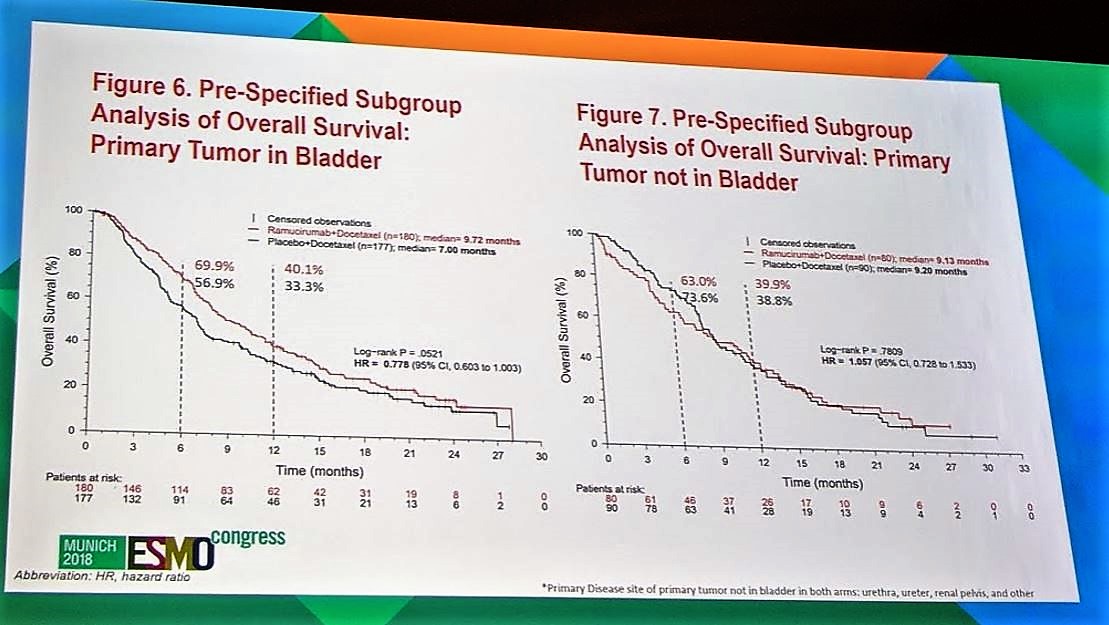
There was a pre-specified subgroup analysis of overall survival for patients who had their primary tumor in the bladder vs primary tumor, not in the bladder (ureter, renal pelvis). This was done as it has been established that patients who have primary tumors outside of the bladder trend towards having worse prognosis3. In this analysis, again, while patients treated with ramucirumab + docetaxel had numerically better median overall survival (9.72 months vs 7.00 months), this was not statistically significant (p=0.0521). Exploratory biomarker analysis with PD-L1 expression did not provide additional information regarding which patients would benefit the most with ramucirumab+docetaxel.
Current second-line options for metastatic UC after progression on platinum-based chemotherapy include immune checkpoint blockade with various PD-1 and PD-L1 inhibitors (pembrolizumab - Keynote-045, atezolizumab - IMvigor211, nivolumab - CheckMate 275 trial, avelumab - JAVELIN, durvalumab - MEDI4736) as well as chemotherapy including docetaxel, vinflunine, and pemetrexed. This study met its primary endpoint of improving progression free survival but unfortunately, ramucirumab + docetaxel does not improve overall survival compared with docetaxel alone. There were limited patients treated after checkpoint inhibition so the authors are not able to comment on this subset of patients at this time. Hopefully, future biomarker work will be able to help us distinguish which patients will benefit the most from chemotherapy vs immunotherapy, or perhaps even combination therapy.
Presented By: Daniel P. Petrylak, MD, Yale Cancer Center, New Haven, Connecticut
Written By: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, Twitter: @TheRealJasonZhu at the 2018 European Society for Medical Oncology Congress (#ESMO18), October 19-23, 2018, Munich Germany
References:
1. Crew JP, O'brien T, Bicknell R, Fuggle S, Cranston D, Harris AL. Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. The Journal of urology 1999;161:799-804.
2. Petrylak DP, Tagawa ST, Kohli M, et al. Docetaxel as monotherapy or combined with ramucirumab or icrucumab in second-line treatment for locally advanced or metastatic urothelial carcinoma: an open-label, three-arm, randomized controlled phase II trial. Journal of Clinical Oncology 2016;34:1500-9.
3. Moschini M, Shariat SF, Rouprêt M, et al. Impact of Primary Tumor Location on Survival from the European Organization for the Research and Treatment of Cancer Advanced Urothelial Cancer Studies. The Journal of Urology 2018;199:1149-57.