(UroToday.com) The treatment for metastatic hormone-sensitive prostate cancer plenary session at the European Association of Urology (EAU) 2021 annual meeting included a presentation by Dr. Noel Clarke discussing systemic treatments and how to choose the right treatment for the right patient.
The importance of metastatic sites in prostate cancer was assessed in a SEER database analysis of 17,167 men with metastatic prostate cancer, of which 63.1% presented with only bone metastases, while bone and non-regional lymph node metastases co-occurred in 8.9% of the cohort.1 Landmark analyses limited to survivors of ≥6 and ≥12 months showed a significantly increased risk of all-cause mortality for patients presenting with bone plus non-regional lymph node metastases compared with patients with only bone metastases:

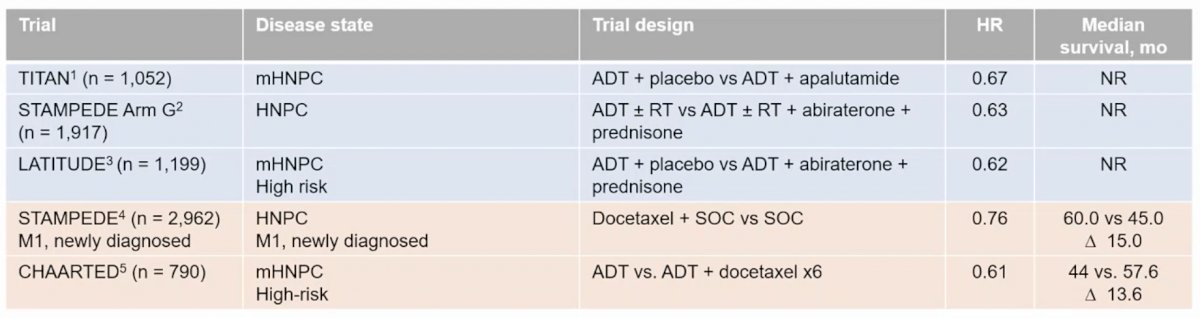
Based on a multitude of large phase 3 randomized clinical trials, combination systemic treatment is now the standard of care for mHSPC. Dr. Clarke notes that one of the keys to choosing the right treatment for the right patient is timing, and in the mHSPC disease state this means early systemic treatment. Based on the data for apalutamide, abiraterone, and docetaxel, the hazard ratios range from HR 0.61-0.76 with median survival ranging from 44 months to not reached:
The importance of disease burden is also key to choosing the right treatment for the right patient, particularly with regards to radiotherapy to the primary site. Data from the STAMPEDE arm H trial assessed the efficacy of radiotherapy to the primary in M1 disease.2 This study randomized 2,061 to either standard systemic treatments (ADT +/- chemotherapy) versus standard systemic treatments (ADT +/- chemotherapy) plus radiotherapy to the primary. There were 819 (40%) men that had a low metastatic burden, 1,120 (54%) had a high metastatic burden, and the metastatic burden was unknown for 122 (6%). Radiotherapy improved failure-free survival (HR 0.76, 95% CI 0.68-0.84) but not OS (HR 0.92, 95% CI 0.80-1.06). In a prespecified subgroup analysis, patients receiving radiotherapy to the prostate among patients with low metastatic burden, there was a significant improvement in OS (HR 0.68, 95% CI 0.52-0.90).
Data published earlier this year assessed the association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer.3 This was an exploratory analysis of treatment outcomes based on metastatic site and extent within the STAMPEDE trial's metastasis M1 radiotherapy comparison. A total of 1,939 men were included, of which 1,732 (89%) had bone metastases. Bone metastasis counts were associated with overall survival and failure-free survival benefit from prostate radiotherapy. Survival benefit decreased continuously as the number of bone metastases increased, with benefit most pronounced up to 3 bone metastases. Further analysis based on subgroups showed that the magnitude of benefit from the addition of prostate radiotherapy was greater in patients with low metastatic burden with only nonregional lymph nodes (M1a) or 3 or fewer bone metastases without visceral metastasis (HR for OS 0.62, 95% CI 0.46-0.83; HR for FFS 0.57, 95% CI, 0.47-0.70) than among patients with 4 or more bone metastases or any visceral/other metastasis (HR for OS 1.08, 95% CI, 0.91-1.28; interaction p = 0.003; HR for FFS 0.87, 95% CI, 0.76-0.99; interaction p = 0.002):

With regards to whether patients should be treated with docetaxel or abiraterone acetate, Dr. Clarke highlighted a STAMPEDE analysis of head-to-head (non-randomized) data from 556 patients.4 With an endpoint of failure-free survival or progression-free survival, there is strong evidence for abiraterone acetate + prednisone, where there is weak evidence when metastatic progression-free survival is the clinical endpoint. For the endpoints of symptomatic skeletal events, cancer-specific survival, and overall survival there is no good evidence for a difference between docetaxel and abiraterone acetate + prednisone.

Finally, Dr. Clarke discussed the importance of disease burden in choosing the right treatment for the patient. In 2019, the STAMPEDE docetaxel trial reported long-term outcomes stratified by metastatic burden for M1 patients.5 Among 1,086 M1 patients randomized, metastatic burden was assessable for 830 (76%0 of patients, including 362 (44%) low and 468 (56%) high metastatic burden. There was good evidence of the benefit of docetaxel over standard of care on OS (HR 0.81, 95% CI 0.69-0.95, p = 0.009) with no evidence of heterogeneity of docetaxel effect between metastatic burden sub-groups (interaction p = 0.827). Analysis of other outcomes found evidence of benefit for docetaxel over standard of care in failure-free survival (HR 0.66, 95% CI 0.57-0.76, p < 0.001) and progression-free survival (HR 0.69, 95% CI 0.59-0.81, p < 0.001) with no evidence of heterogeneity of docetaxel effect between metastatic burden sub-groups (interaction p > 0.5 in each case).
Dr. Clarke then made the following concluding statements regarding de-novo mHSPC for his presentation:
- Early combination therapy is the standard of care
- Radiotherapy to the primary site is beneficial in low-burden disease
- Systemic treatment is effective irrespective of burden/risk stratification
- Chemotherapy and NHA appear to be equally effective
- Quality of life may be better in the long-term when using NHAs
Presented by: Noel W. Clarke, MBBS, FRCS, ChM, Christie and Salford Royal NHS Foundation Trusts, Manchester, UK
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Ali A, Hoyle A, Mistry H, et al. Importance of non-regional lymph nodes in assigning risk of primary metastatic prostate cancer. BJU Int. 2019 Jan;123(1):65-73.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Ali A, Hoyle A, Haran AM, et al. Association of Bone Metastatic Burden with Survival Benefit from Prostate Radiotherapy in Patients with Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Onc 2021 Apr 1;7(4):555-563.
- Sydes MR, Spears MR, Mason MD, Clarke NW, Dearnaley DP, de Bono JS, et al. Adding abiraterone or docetaxel to long-term hormone therapy for prostate cancer: directly randomised data from the STAMPEDE multi-arm, multi-stage platform protocol. Ann Oncol. 2018;29:1235-48.
- Clarke NW, Ali A, Ingleby FC, et al. Addition of docetaxel to hormonal therapy in low- and high-burden metastatic hormone sensitive prostate cancer: long-term survival results in the STAMPEDE trial. Ann Oncol 2019 Dec 1;30(12):1992-2003.