(UroToday.com) The European Association of Urology (EAU) 2021 Virtual Meeting included a joint session of the EAU and the Advanced Prostate Cancer Consensus Conference and a discussion regarding current gaps in the evidence, specifically optimal management of cN1 patients. Participants in this discussion included Drs. Ricardo Mestre, Alberto Briganti, Piet Ost, Karim Fizazi, and Peter Albers.
Dr. Mestre started the discussion by highlighting a clinical case of a 65-year-old farmer, non-smoker, with regular alcohol consumption (1L of beer/day) and a urological history of BPH treated with a TURP in 2016. His medical history included well-controlled hypertension. He presented with a PSA of 13 ng/mL, IPSS score of 5/35, and an enlarged prostate with bilateral diffuse induration (cT2c) on DRE. A mpMRI of the prostate suggested right potential extraprostatic extension. A subsequent prostate biopsy showed prostate adenocarcinoma, Gleason 5+4 in 8 out of 10 biopsies, with a ki-67 proliferation index of 65-70%. Staging studies included an abdominal CT scan that confirmed two enlarged pelvic lymph nodes (right internal iliac and right obturator, both with 2cm of short axis diameter), a bone scan that was negative, and a PSMA PET that showed no distant metastases and confirmed the two enlarged pelvic lymph nodes and prostate lesion. Dr. Mestre notes that there are several treatment options for this patient, including:
- ADT for 24-36 months?
- Locoregional treatment (recommended by 98% of panelists at APCCC 2019)?
- Radiotherapy to the prostate with ADT?
- Radiotherapy to the prostate, pelvis, and boost for PSMA-positive lymph nodes plus ADT
- Radical prostatectomy plus extended pelvic lymphadenectomy?
- ADT + systemic treatment?
- ADT plus abiraterone
- ADT plus docetaxel
Dr. Mestre notes that the EAU guidelines suggest that there is weak evidence to offer patients with cN1 disease a local treatment (either radical prostatectomy or intensity-modulated radiotherapy plus image-guided radiotherapy) plus long-term ADT.
Dr. Alberto Briganti then discussed the role of surgery for cN1 prostate cancer. He notes that there is only retrospective evidence for the role of surgery in cN1 disease, studies are at high risk of patient selection bias (there are no standardized indications for surgery), there is no standardized extent of pelvic lymph node dissection/use of multi-modal approaches, and indications are mainly based on the use of conventional imaging. As previously mentioned by Dr. Mestre and highlighted again by Dr. Briganti, the EAU guidelines give a weak recommendation for offering patients with cN1 disease a local treatment (either radical prostatectomy or intensity-modulated radiotherapy plus image-guided radiotherapy) plus long-term ADT. Dr. Briganti highlighted the pros and cons regarding the implications of surgery with the following table:
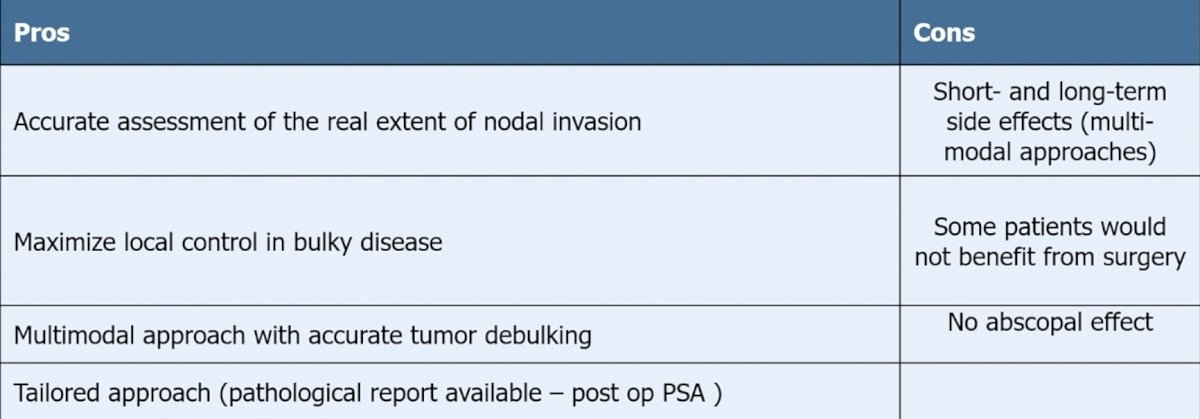
A systematic review previously assessed the role of definitive local treatment in patients with clinically lymph-node positive prostate cancer [1], noting four studies that compared the use of radiotherapy ± ADT versus ADT alone, whereas one study compared any form of local therapy ± ADT versus ADT alone. Overall, the use of radiotherapy and, generally, any form of local treatment was associated with an OS as well as a cancer-specific survival benefit over ADT alone, without any clear superiority shown either by radical prostatectomy ± ADT or by radiotherapy ± ADT.
Dr. Briganti notes that there is also an oncologic control for the rationale for surgery in patients with cN1 disease. Seisen and colleagues [2] used the NCDB (2003-2011) to retrospectively identify 2,967 individuals who received local treatment ± ADT versus ADT alone for cN1 prostate cancer. Overall, 1987 (67%) and 980 (33%) patients received local treatment ± ADT and ADT alone, respectively. In the local treatment ± ADT group, 751 (37.8%) and 1236 (62.2%) patients received radical prostatectomy ± ADT and radiotherapy ± ADT, respectively. When comparing radical prostatectomy ± ADT versus radiotherapy ± ADT, instrumental variable analysis showed no significant difference in overall mortality-free survival between the two treatment modalities (HR 0.54, 95% CI 0.19-1.52, p=0.20):

As always, patient selection for radical prostatectomy for cN1 positive patients is of the utmost importance. The following algorithm summarizes patient outcomes by risk groups [3]:

Dr. Briganti concluded his presentation with the following take-home messages:
- Surgery is a treatment option for selected cN1 men (provided extensive surgery)
- An extended lymph node dissection at the time of radical prostatectomy is the gold standard for staging (up to 20% of cN+ patients eventually are pN0)
- The ideal candidate is still to be determined
- Multi-modal approach can be tailored according to each patient profile
- The lack of high-level evidence still represents a major drawback
Dr. Piet Ost then discussed the role of radiotherapy in patients with cN1 disease. Dr. Ost started by emphasizing that radiotherapy is standard of care for conventionally staged prostate cancer from cN0, cN1, to cM1.
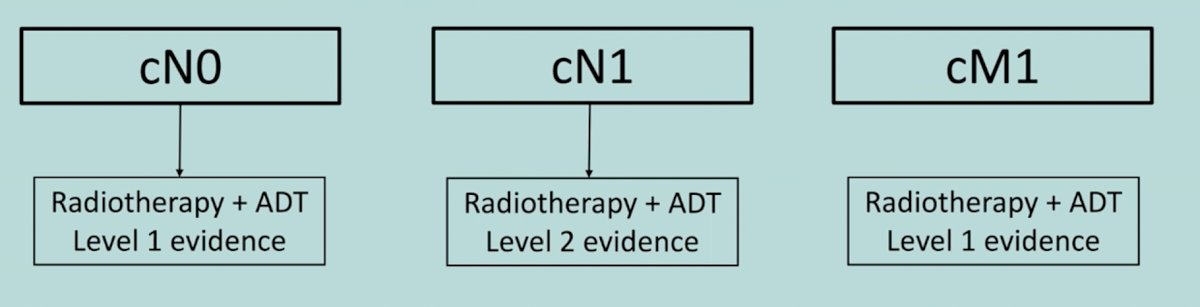
For cN0 high-risk patients, radiotherapy + ADT has been established as superior to ADT alone. For cM1 patients, the STAMPEDE arm H data [4] showed an 8% absolute OS benefit among low burden disease patients (HR 0.68, 95% CI 0.52-0.90). Low burden is defined as patients with only non-regional lymph nodes or 3 or fewer bone metastases with or without non-regional lymph nodes regardless of axial or extra axial location and without any visceral/other metastases. For cN1 patients, Dr. Ost highlighted failure-free survival in the control arm of the STAMPEDE trial and the impact of radiotherapy for these patients. Among 721 men with newly diagnosed M0 disease, there were 40 deaths (31 owing to prostate cancer) with 17 months' median follow-up. Time to failure-free survival was worse in patients with N+ disease (HR 2.02, 95% CI 1.46-2.81) than in those with N0 disease. Furthermore, failure-free survival outcomes favored planned use of radiotherapy for patients with both N0M0 (HR 0.33, 95% CI 0.18-0.61) and N+M0 disease (HR 0.48, 95% CI 0.29-0.79):

Dr. Ost concluded by noting that PSMA shifts patients from one category to another (cN0 cN1 cM1), but regardless of category radiotherapy is needed either way.
Dr. Karim Fizazi then discussed the role of systemic therapy for cN1 positive patients, starting by highlighting that there are >100 randomized controlled trials for high-risk localized prostate cancer, and >40 trials for metastatic cancer, but there are essentially no clinical trials for patients with cN0 disease. Looking back at the original Messing trial published in 1999, this trial (although small) showed that immediate ADT improves overall survival among patients with pN+ disease. In the STAMPEDE abiraterone trial [6], there was a subset of patients (n=384) that were N+M0, of which 314 men received radiotherapy and 70 men did not receive radiotherapy. With an outcome of recurrence free survival, cN+ patients treated with ADT + abiraterone versus ADT alone had improved outcomes:

There is also evidence for docetaxel in this disease space. At the ESMO 2018 annual meeting, 12-year follow-up results of the GETUG-12 phase 3 trial of docetaxel in high-risk localized prostate cancer were presented, with the following figure highlighting the trial schema:

This trial found that there was a significant difference for relapse-free survival, 11.6 months versus 8.1 months (HR 0.71, 96%CI 0.55 0.55-0.93) favoring the docetaxel arm. For relapse-free survival, those patients who benefited the most were those with high-risk features as expected (node positive, PSA>20).
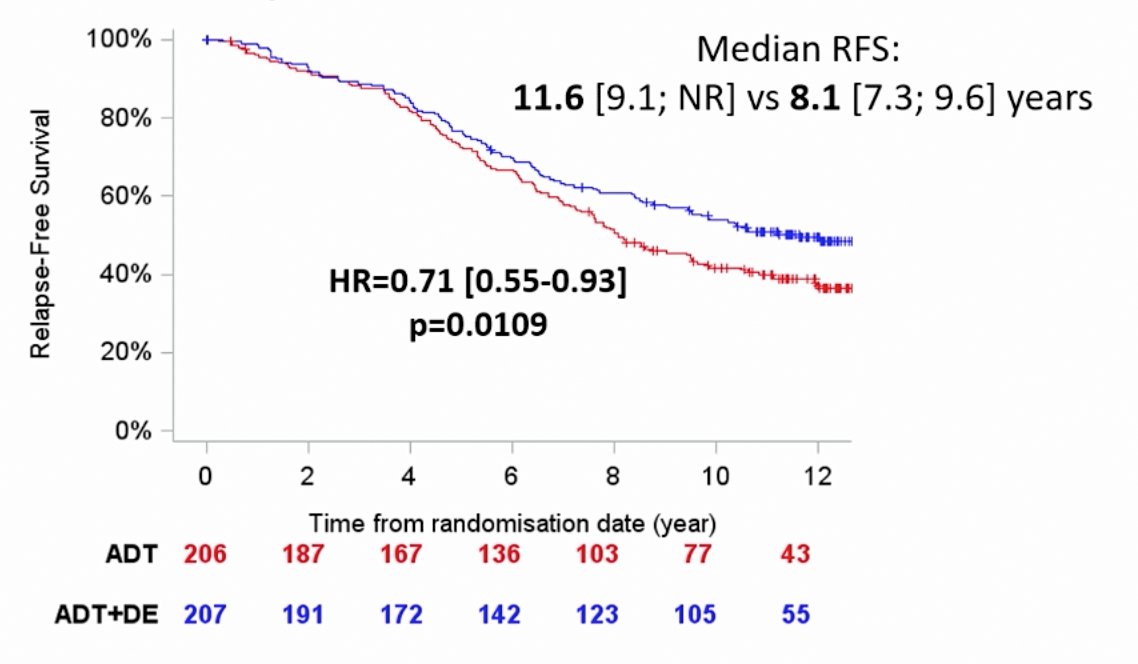
In a subgroup analysis of GETUG-12, pN1 or pN2 patients also benefited from the addition of docetaxel to ADT (HR 0.64, 95% 0.43-0.95).
Dr. Fizazi concluded his presentation with the following summary points:
- There is insufficient level of evidence to appropriately delineate the optimal treatment of cN1 prostate cancer
- This disease space is in need of a randomized clinical trial and the use of next-generation imaging
- For the case being discussed, Dr. Fizazi states that he would recommend either long-term ADT (3 years?) or abiraterone (2 years?) and external beam radiotherapy to the prostate and lymph nodes if possible
Dr. Peter Albers closed this discussion by summarizing the cN1+ presentations with some final thoughts. Frist, the optimal management for patients with cN1 prostate cancer should be to aim for a cure, or at the very least for long term overall survival and no toxicity. It is important to note, that regardless of the treatment the patient/physician choose, most men will likely live for many years, with the majority of low risk patients (PSA doubling time > 1 year, ISUP 1-3 disease) living for more than 10 years. For high risk men (PSA doubling time <1 year, ISUP 4-5), the five-year metastasis free survival rate is 87%, and Dr. Albers notes that these men may be the group that we have to take care of. He hopes that in the near future we may be able to identify very high-risk cN1 patients based on molecular features.
Long-term outcomes of patients treated with salvage lymph node dissection for nodal recurrence until recently were essentially unknown. Bravi et al. [7] undertook a multi-institutional approach to assess these long-term outcomes, including 189 patients who experienced PSA rise and nodal-only recurrence after radical prostatectomy and underwent salvage lymph node dissection at 11 centers between 2002 and 2011. Recurrences were detected with either 11C-choline or 68Ga PSMA. The primary outcome was cancer-specific mortality, and the secondary outcomes were overall mortality, clinical recurrence, biochemical recurrence, and androgen deprivation therapy (ADT)-free survival after salvage lymph node dissection. There were 110 and 163 patients experienced clinical recurrence and biochemical recurrence, respectively, with clinical recurrence-free and biochemical recurrence-free survival at 10 years of 31% and 11%, respectively. After salvage lymph node dissection, a total of 145 patients received ADT, with a median time to ADT of 41 months:

Importantly, additional therapy was warranted in >60% of patients within 6 months of salvage lymph node dissection. At a median follow-up for survivors of 87 (IQR 51 to 104) months, 48 patients died, of which 45 died from prostate cancer. At multivariable analyses, patients who had PSA response after salvage lymph node dissection (HR 0.45; p = 0.001), and those receiving ADT within 6 months from salvage lymph node dissection (HR 0.51; p = 0.010) had lower risk of death from prostate cancer.
For cN1 positive patients, Dr. Albers personal management is to stage the patient with PSMA PET/CT in high risk patients with biochemical recurrence (PSA >0.5). If the patient has <= 2 nodes positive he will discuss local treatment, either radioguided robotic salvage lymph node dissection or targeted radiotherapy. If the patient has multiple nodes, positivity in an M1a location, or further PSA progression after salvage lymph node dissection his systemic therapy of choice is intermittent ADT.
Presenter by:
- Ricardo Pereira Mestre, Oncology Institute of Southern Switzerland, Bellizona, Switzerland
- Alberto Briganti, Urological Research Institute, IBCAS San Raffaele Scientific Institute, Milan Italy
- Piet Ost, Ghent University, Ghent, Belgium
- Karim Fizazi, Institut Gustave Roussy, Universite Paris-Saclay, Villejuif, France
- Peter Albers, University of Dusseldorf, Dusseldorf, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.
References:
- Ventimiglia E, Seisen T, Abdollah F, et al. A systematic review of the role of definitive local treatment in patients with clinically lymph node-positive prostate cancer. Eur Urol Oncol. 2019 May;2(3):294-301.
- Seisen T, Vetterlein MW, Karabon P, et al. Efficacy of local treatment in prostate cancer patients with clinically pelvic lymph node-positive disease at initial diagnosis. Eur Urol 2018 Mar;73(3):452-461.
- Gandaglia G, Soligo M, Battaglia A, et al. Which patients with clinically node-positive prostate cancer should be considered for radical prostatectomy as part of multimodal treatment? The impact of nodal burden on long-term outcomes. Eur Urol. 2019 May;75(5):817-825.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomized controlled phase 3 trial. Lancet 2018 Dec 1;392(10162):2353-2366.
- James ND, Spears MR, Clarke NW, et al. Failure-free survival and radiotherapy in patients with newly diagnosed nonmetastatic prostate cancer: Data from the patients in the control arm of the STAMPEDE trial. JAMA Oncol. 2016 Mar;2(3):348-357.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Engl J Med. 2017;377(4):338-351.
- Bravi CA, Fossai N, Gandaglia G, et al. Long-term Outcomes of Salvage Lymph node dissection for nodal recurrence of prostate cancer after radical prostatectomy: Not as Good as Previously Thought. Eur Urol 2020 Nov;78(5):661-669.