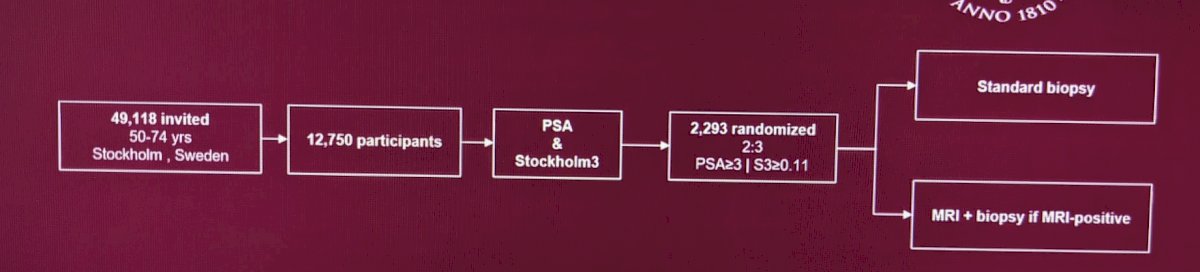
- The STHLM3RS marker was created at the Karolinska Institutet, a world-leading medical university, and Thermo Fisher Scientific. It is a blood-based test including analyses of PSA and four other proteins, 101 genetic markers (single nucleotide polymorphisms), and clinical information (age, family history, earlier biopsies, and use of 5-alpha reductase inhibitors).
- The MRI was a 1.5/3T MRI (mostly 1.5T), 16-minute protocol without DCE
- Biopsies were 3-4 targeted biopsies of PIRADS3-5 lesions, standard bx was 10-12 cores
Analysis was an intention-to-treat (ITT).
Baseline characteristics stratified by arm are listed below:
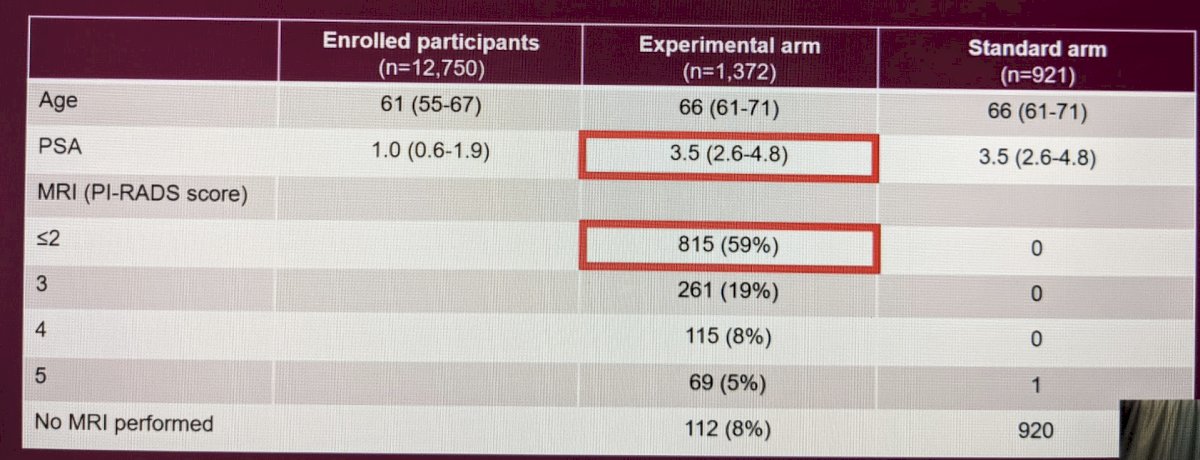
Most MRIs were essentially negative (59%). Median PSA was 3.5 in both arms.
Ultimately the MRI targeted biopsy pathway was non-inferior for detection of significant prostate cancer and was associated with the lower detection of insignificant prostate cancer. Results are seen below:
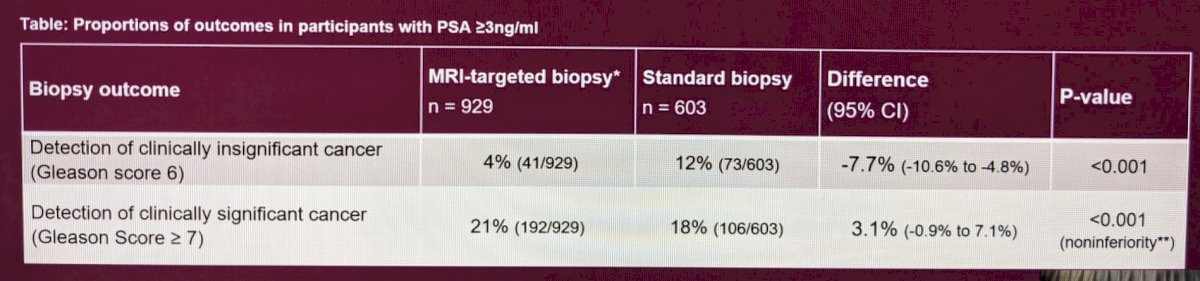
In fact, it detected more clinically significant prostate cancer (21% vs. 18%) and much less insignificant prostate cancer (4% vs. 12%).
The Stockholm3 test, similar to prior studies, discriminated for significant prostate cancer better than PSA (AUC 0.76 vs. 0.60).
Looking at the different diagnostic strategies and various outcomes below:
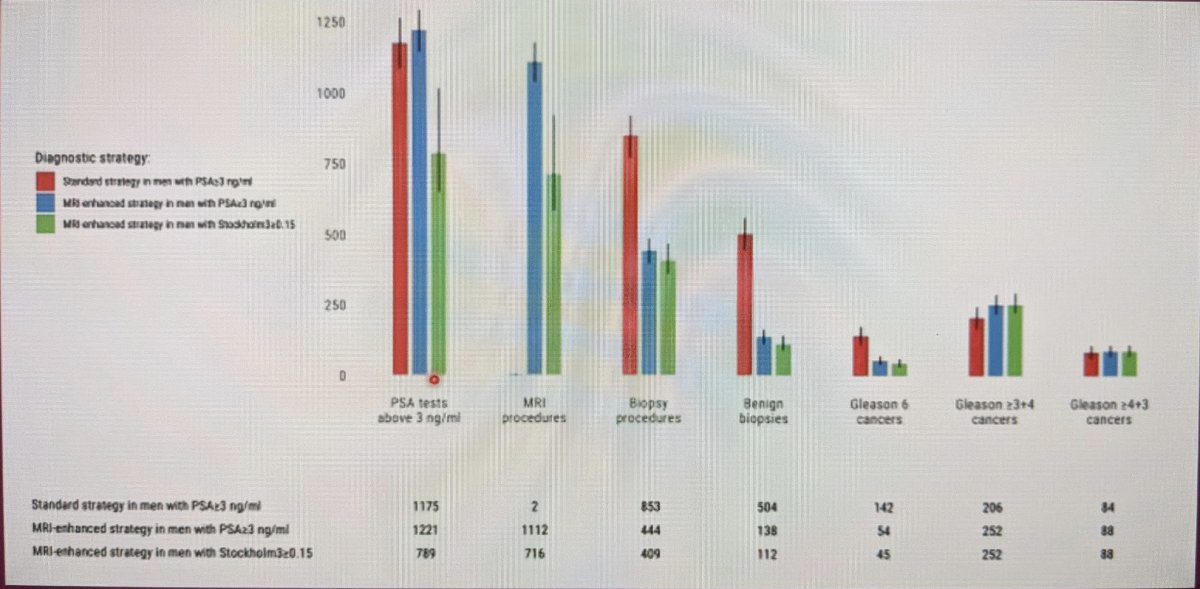
- Strategy 3 (MRI enhanced in men with Stockholm3 score >0.15) had less MRI procedures, less biopsies procedures, better detection of clinically significant prostate cancer, and less detection of insignificant prostate cancer
- MRI with targeted and systematic biopsy in MRI positive men was non-inferior to standard biopsy for detecting clinically significant prostate cancer in a population-based screening-by-invitation setting and markedly reduced detection of clinically insignificant cancer
- The Stockholm3 test can inform risk ratification before MRI and targeted biopsies in prostate cancer screening
- Combining the Stockholm3 test with an MRI target biopsy approach for prostate cancer screening decreases over detection while maintaining detection of significant cancer
Presented by: Tobias Nordstrom, MD, PhD, Karolinska Institutet, Stockholm, Sweden
Written by: Thenappan (Thenu) Chandrasekar, MD – Urologic Oncologist, Assistant Professor of Urology, Sidney Kimmel Cancer Center, Thomas Jefferson University, @tchandra_uromd on Twitter during the 2021 European Association of Urology, EAU 2021- Virtual Meeting, July 8-12, 2021.


