His primary work has been with plasma DNA – blood collected from the patient is spun down to remove cells, and then DNA is extracted from the plasma. Naturally, this is DNA from both the tumor and normal tissue. ctDNA (circulating tumor DNA, DNA from the tumor) can encompass anywhere from <1% to >90% of the plasma DNA fraction. Compared to CTC’s, plasma free DNA is amenable to frequent and repeated sampling in real-time.
One of the first hurdles was to find a way to identify the ctDNA from normal patient DNA. To do so, gene mutations that occur early in cancer genesis and persistent through disease progression was important. They identified two deletions, 21q and 8p, that are well preserved in PCa, and can thereby serve as the “standard” for additional mutations to be compared against. In the slide below, he demonstrates how quantifying additional mutations against these standards can help identify up and down-regulation over disease progression, in response to different treatments.
They also utilized heterozygous SNP’s to quantify the abundance of mono-allelic deletions, which further helped delineate tumor content in the specimen.
They have begun to produce work demonstrating the clinical utility of ctDNA. In a paper from 2015 (Romanel Sci Transl Medicine 2015), they demonstrated that ctDNA fractions > 18% portended worse OS and PFS in progressing CRPC patients. 18% was the median of the group, and was used as the cutoff.
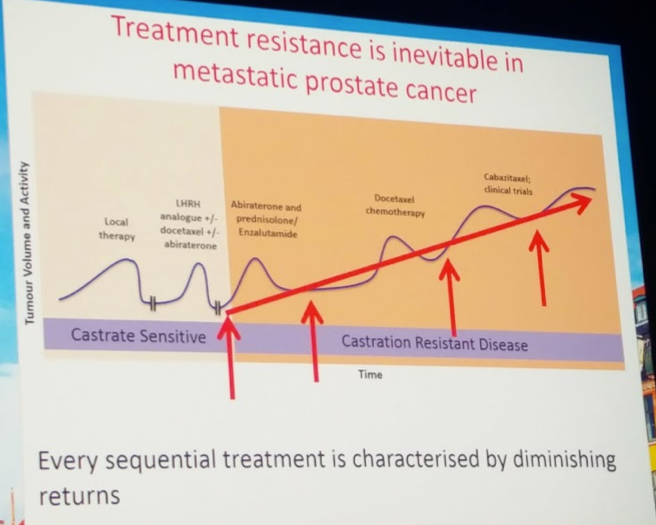
He then began to address the evaluation of AR-targeted therapy resistance. Identifying these mechanisms is an unmet need. Better predictors of primary non-responders and eventual failures of therapy can help in the decision making process.
AR copy number gain or somatic point mutations are not usually detected prior to hormonal manipulation, but are highly prevalent after ADT. By tracking AR point mutations in abiraterone-treated patients, they were able to identify two substitutions that were associated with progression. L702H and T878A were present in about 16% of patients and were associated with progression of disease. Clinically, both pre- and post-chemo abiraterone treated patients were stratified based on AR normal or AR gain – in both situations, AR gain mutation patients had worse OS and PFS. This was externally validated in a cohort from the Spanish PREMIERE trial.
Importantly, taxane efficacy was not affected by AR status. Hence, in these patients, taxane therapy may be preferred.
This work is very exciting and augments much of the other work looking for biomarkers in this disease space!
Presented by: Professor Gerhardt Attard, MD, PhD, FRCP, University College London, London, UK
Written by: Thenappan Chandrasekar, MD Clinical Fellow, University of Toronto, twitter: @tchandra_uromd at the 2018 European Association of Urology Meeting EAU18, 16-20 March, 2018 Copenhagen, Denmark
Further Related Content:
Watch: Biomarker Development in Advanced Prostate Cancer- Alicia Morgans