In this study, the authors utilized a population-based approach to address the role of adjuvant TT in the management of RCC. Specifically, they utilized the SEER database to identify all patients with RCC from 2006 to 2013; these patients were then stratified by the presence of metastatic disease at the time of diagnosis (cM0 / cM1). cM0 patients following surgical excision were stratified into low and high-risk based on a combination of ASSURE and S-TRAC criteria.
Based on above criteria, 79,926 patients were included - 71,682 cM0 and 8,244 cM1 patients. The median follow-up for the entire cohort was 40.1 months. Of 31,453 patients with histologic grade data, 18,328 and 13,125 were low- and high-risk cM0, respectively.
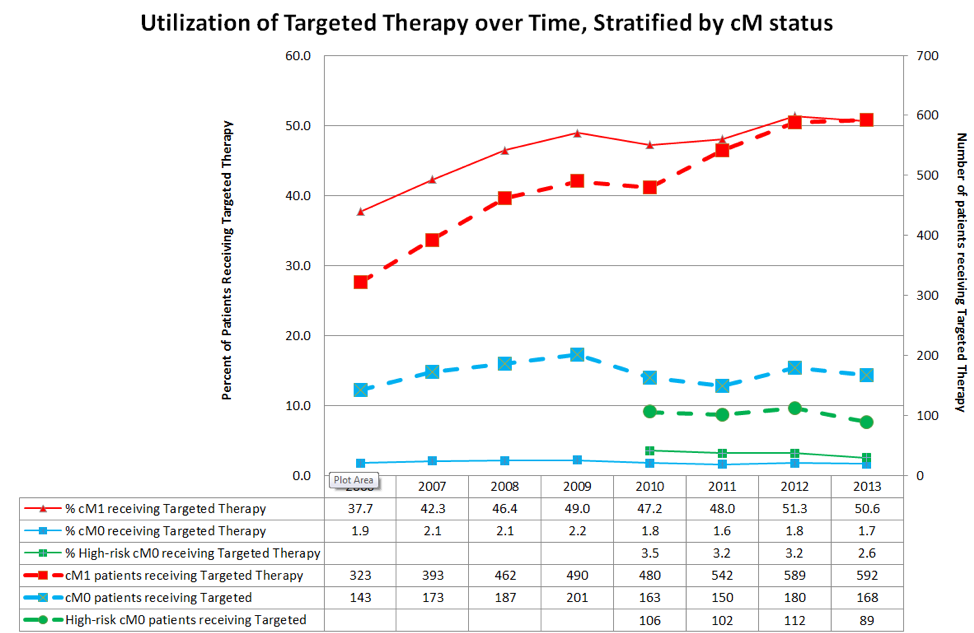
TT utilization in cM1 patients peaked at 50.6% and was associated with reduced CSM (HR 0.73, p<0.01). In contrast, TT utilization (which was presumed salvage therapy) never exceeded 2.2% in the entire cM0 cohort and 3.5% in the high-risk cM0 cohort. On competing risks analysis, TT receipt was associated with increased CSM in all cohorts, including subset analyses – though this probably represented selection bias for worse disease.
Importantly, however, Kaplan-Meier survival analyses demonstrate that other-cause mortality exceeds cancer-specific mortality in the cM0 population.
Using this inferred analysis from population-level data, the authors conclude that, when compared to the cM1 patients, TT receipt in cM0 patients does not provide any cancer-specific survival benefit, even in the small percentage of patients that eventually progress to metastatic disease. Competing risks mortality (other-cause mortality) further limit any potential benefit in this population, as these patients are more likely to die of other causes than RCC.
Presented by: Thenappan Chandrasekar, University Health Network, University of Toronto, Dept. of Surgical Oncology, Division of Urologic Oncology, Toronto, Canada
Written by: Thenappan Chandrasekar, MD Clinical Fellow, University of Toronto, twitter: @tchandra_uromd at the 2018 European Association of Urology Meeting EAU18, 16-20 March, 2018 Copenhagen, Denmark


