Following his introduction and talks from Dr. Cooperberg on Pre-diagnostic testing and germline testing, Dr. Wagner presented on how to incorporate testing with Decipher and ProMark at the time of diagnosis. At this junction in the natural history of prostate cancer, genomic testing can add nuance to questions regarding whether to pursue active surveillance or definitive treatment and, among those who opt for treatment, whether to intensify treatment.
Genomic testing can add nuance to the data available from standard clinicopathologic characteristics. Doing so allows for the personalization of treatment recommendations on the basis of the potential for adverse outcomes.
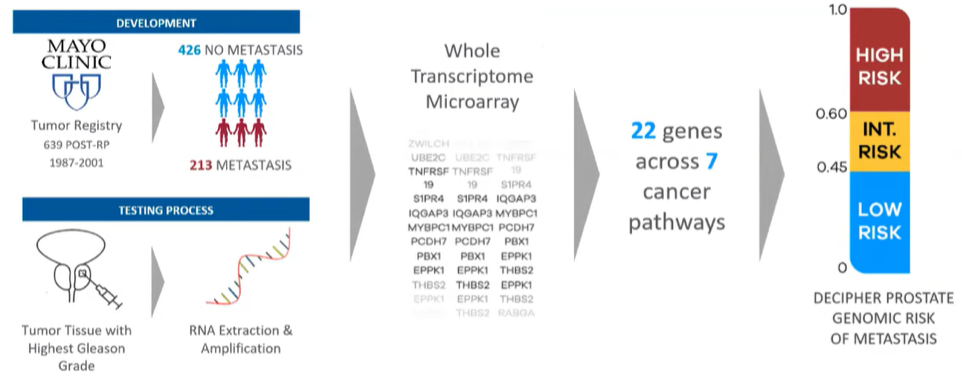
Dr. Wagner began by describing both the derivation and clinical data supporting the Decipher test. Notably, Decipher was designed on the basis of metastasis-free survival, which has in other datasets, been shown to be the strongest surrogate for overall survival. The Decipher test was originally derived on the basis of 639 patients who underwent radical prostatectomy at the Mayo Clinic. RNA was extracted and amplified from tumor tissue from the highest grade lesion within the prostate. Following whole transcriptome microarray analysis, 22 relevant genes across seven cancer pathways were identified and used to derive the Decipher risk score.
Based on univariable models, Decipher outperforms NCCN risk stratification for the prediction of metastasis.
Dr. Wagner then walked through a sample Decipher Prostate Biopsy report, highlighting results including the Decipher Score, the risk of metastasis, the risk of prostate cancer mortality, the risk of adverse pathology, and the risk comparison compared to other patients with comparable NCCN risk stratification. This information can then be used to guide treatment decisions.
In particular, Dr. Wagner highlighted the potential for genomic testing with Decipher to identify men with a low risk of developing metastatic disease who would therefore be potentially good candidates for active surveillance. However, this premise must be considered while acknowledging that Decipher was derived among men who had undergone radical prostatectomy. Further, in the converse, Decipher testing may detect men with low-risk disease according to clinicopathologic criteria who harbor biologically aggressive tumors who may benefit from active treatment.
Dr. Wagner then used a number of hypothetical examples to highlight the ability of genomic testing to provide treatment guidance in equivocal situations, such as patients with high volume GG1 disease. Further, among patients who are opting for treatment, understanding the underlying disease biology may contribute to treatment decision-making. For example, while he marginally uses this information in surgical decision making, it may contribute to decisions regarding whether to administer ADT with radiotherapy and if so, for how long. In particular, those with low risk according to their Decipher assay may be spared ADT while those with higher risk may warrant longer durations of therapy. However, it is important to understand that Decipher is prognostic and these data do not prove a predictive ability to guide treatment.
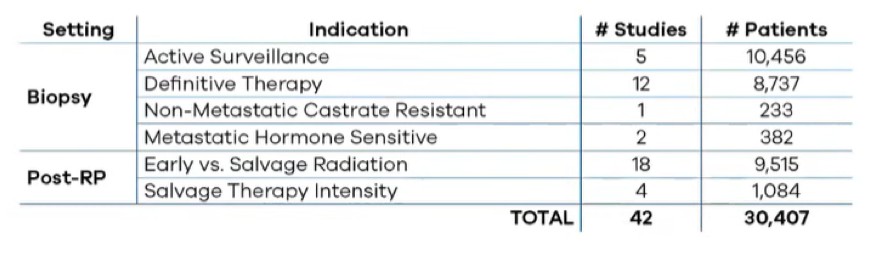
Dr. Wagner then highlighted a recent systematic review of 42 studies and more than 30,000 patients assessing the evidence underlying Decipher in prostate cancer. These data demonstrated that the Decipher score is independently prognostic for overall survival, metastasis, prostate cancer-specific mortality, adverse pathology, and biochemical failure. Further, Decipher is more accurate in risk stratifying prostate cancer than clinicopathologic variables alone and this information can impact treatment decisions.
In addition to these data, there are numerous ongoing research efforts including retrospective analyses of existing phase III trials and prospective phase II and III studies specifically examining its role in this disease.
Dr. Wagner then moved to discuss the role of ProMark. This, like Decipher, is a prognostic test although instead of relying on an RNA expression signature, ProMark is based on an eight-protein signature. The use of a protein signature obviates the need for tumor microdissection and RNA extraction as well as allowing for direct measurement on prepared biopsy slides. Further, such proteomic assessment addresses intra-tumoral heterogeneity to a greater extent than genomic tests. As with other assays discussed in this course, ProMark is supported by a body of peer-reviewed literature including four multi-center studies including approximately 1200 patients, including platform validation studies, clinical validity studies, clinical utility studies, and supportive economic impact analysis.
In Dr. Wagner’s view, the use of ProMark shifts the needle towards surveillance. He highlighted a hypothetical example report to emphasize the actionability of reported data.
Highlighting a variety of examples across the spectrum from very low risk to intermediate-risk disease, he showed how the use of ProMark can result in individualized risk predictions. As highlighted in the figure below, the difference between individualized predictions and the overall group risk are quite small for those with very low-risk disease, thus, testing is of minimal utility in this group. In contrast, differences may be much larger in those with low-risk disease and intermediate-risk disease.
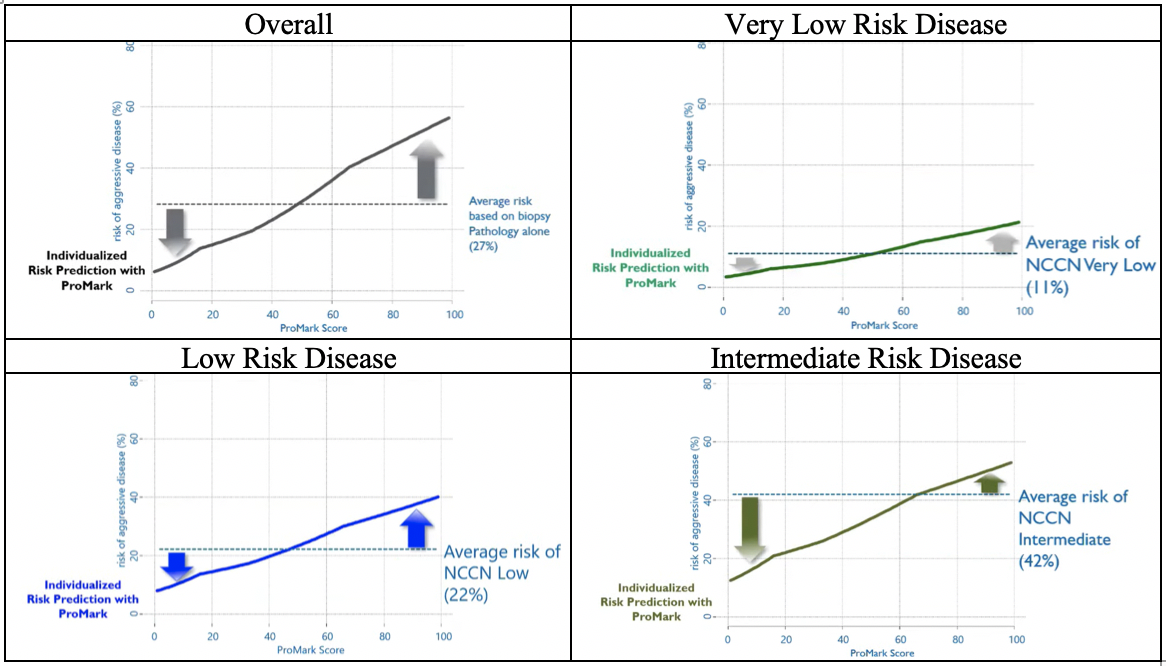
In general, Dr. Wagner suggested that, as shown in the figures above, patients are likely to be identified as having lower risk than their initial stratification would suggest. Thus, the use of ProMark often supports the de-escalation of therapy.
In real-world clinical experience, among those with Gleason 3+3 disease, more than 55% of patients undergoing ProMark testing were identified as having lower risk characteristics while a little more than 15% were found to have high-risk protein signatures. Among those with Gleason 3+4 disease, approximately one-third of patients had a low-risk protein signature while more than 30% had a high-risk signature.
Presented by: Joseph Robert Wagner, MD, Chief, Urology, Hartford Hospital & Director, Robotic Surgery, Hartford Hospital
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter during the AUA2021 May Kick-off Weekend May 21-23


