When trying to define nmCRPC patients, we need to identify them correctly, determine their prognosis, strategize their appropriate treatment options, and understand their treatment implications. There is a key role for PSA doubling time in the determination of prognosis in nmCRPC patients, and the correlation of PSA doubling time to the risk of metastatic disease is not linear, as can be seen in figure 1.
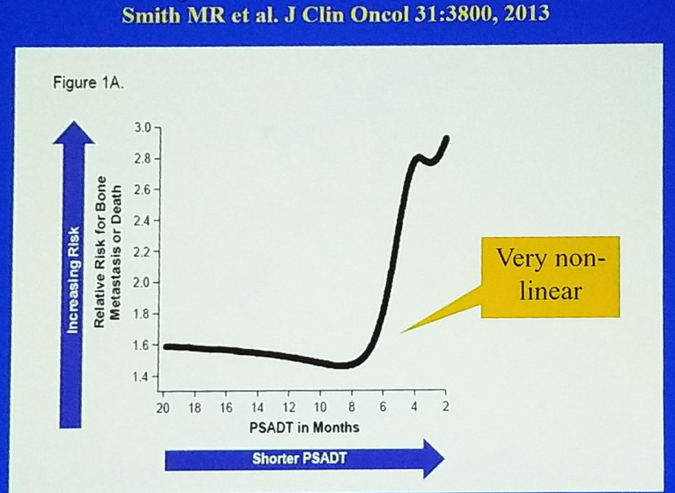
Figure 1 – Prognosis in patients with non-metastatic CRPC: a key role for PSA doubling time
The therapeutic options that are available for nmCRPC patients include:
- No therapy and surveillance only
- Systemic therapies:
b. Newer hormonal therapies – apalutamide, enzalutamide, is abiraterone effective?
- Non-systemic therapies – stereotactic body radiotherapy (SBRT) for molecular staged oligometastatic disease?
Both SPARTAN and PROSPER specifically demonstrated a significant benefit to both apalutamide and enzalutamide in MFS, time to PSA progression, and time to symptomatic progression. There was a clear trend towards improved overall survival for the apalutamide arm and the enzalutamide arm in SPARTAN and PROSPR, respectively, but it was not statistically significant in both trials. Importantly, the adverse effects in the SPARTAN trial demonstrated a high percentage of rash (24%), falls (16%), fractures (12%), and hypothyroidism (8%) for an unknown reason. In the PROSPER trial, treatment discontinuation and deaths due to adverse events were slightly higher in the enzalutamide arm (9% vs. 6%, and 3% vs. 1%, respectively). As a result of the SPARTAN and PROSEPR trials, apalutamide was FDA approved for the setting of nmCRPC patients, and the FDA had broadened the label for enzalutamide to include nmCRPC without regard to PSA doubling time, respectively. A nice table summarizing and comparing the adverse effects of enzalutamide, apalutamide, and abiraterone was created by the institute for clinical and economic review, as shown in table 1.

Table 1 – Comparison of adverse effects of abiraterone, enzalutamide, and apalutamide
When assessing the subsequent treatments these patients had received following enzalutamide, it was shown that abiraterone after enzalutamide resulted in a PSA response of 3%, but the data for abiraterone after apalutamide is not yet clear. However, it is getting clear that secondary therapies are less effective in general.
Dr. Sartor moved on to discuss the role of genetics in advanced prostate cancer. In a study published in the New England Journal of Medicine in 2016, it was shown that 11.8% of men with metastatic prostate cancer had inherited DNA-repair germline mutations.3 These included BRCA2 in 5.8%, CHEK2 in 1.9%, ATM in 1.6%, BRCA1 in 0.9%, and RAD51d and PALB2 0.4% each. This is a most significant finding that requires further research and contemplation.
The role of “liquid biopsy” (sampling the tumor via the blood stream) using cell free DNA, circulating tumor cells (CTCs), exosomes, RNA and others, needs to be defined. Androgen receptor mutations, amplifications, over-expression, splice variants and additional genetic variants are also extremely important, and their predictive power in the specific setting of nmCRCP is not clear.
The role of DNA repair and poly ADP ribose polymerase (PARP)/Platinum sensitivity needs to be further elucidated. In another New England Journal of Medicine paper, published in 2015, treatment with the PARP inhibitor, olaparib, in patients whose prostate cancers were no longer responding to standard treatments and who had defects in DNA-repair genes, led to a high response rate.4 Furthermore, the role of Platinum in metastatic-CRPC patients needs to be understood better. In a paper published in 2016, three metastatic CRPC patients achieved an exceptional response to platinum chemotherapy, despite disease progression on prior standard therapies.5 Using targeted next-generation sequencing on the primary and metastatic tumors of these patients, it was found that all three of them had biallelic inactivation of BRCA2, a known tumor suppressor gene critical for DNA repair.
Lastly, Dr. Sartor discussed the role of immunotherapy in advanced prostate cancer patients. In May 2017, the FDA awarded pembrolizumab, a known anti PD-1 immune checkpoint inhibitor, accelerated approval for cancers with high microsatellite instability or DNA mismatch repair deficiency. Furthermore, it has been shown that loss of both alleles of CDK12 gene, a protein kinase enzyme that is a member of cyclin-dependent kinase protein family, defines a molecular subtype of metastatic CRPC that is potentially targetable with immune checkpoint inhibitors.6 This metastatic CRPC subtype apparently occurs in 6.9% of advanced prostate cancer patients.
Using the new imaging modalities discussed at the beginning of this talk (PET PSMA and others), we might be able to identify more lesions that potentially could be amenable to treatment with Stereotactic Body Radiation Therapy (SBRT). Moreover, extending the usage of the new PSMA ligands, we might be able to treat lesions identified by PET PSMA with PSMA-Lutetium-177, which will specifically target the lesion identified by PET-PSMA.7
Summarizing this excellent talk by Dr. Sartor, newly discovered data with two agents (apalutamide and enzalutamide) are quite similar and raise the same questions. It is still unknown whether the results demonstrated by these two trials would be different if abiraterone were used. This is especially important due to the fact that abiraterone will soon become generic. We need to further expand our knowledge on the role of biomarkers in selecting the appropriate therapy, and need to elucidate if PSMA scans and PSMA-targeted therapy will change the paradigm yet again.
References:
- Smith MR et al. NEJM 2018
- Hussain M et al. NEJM 2018
- Pritchard CC et al. NEJM 2016
- Mateo J et al. NEJM 2015
- Cheng HH et al. Eur Urol 2016
- Wu YM et al. Cell 2018
- Rahbar et al. JNM 2017
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 70th Northeastern Section of the American Urological Association (NSAUA) - October 11-13, 2018 - Fairmont Royal York Toronto, ON Canada