(UroToday.com) The 2022 American Society for Radiation Oncology (ASTRO) Annual Meeting held in San Antonio, TX between October 23rd and 26th, 2022 was host to a session that addressed studies aimed at improving outcomes for high-risk prostate cancer patients. Dr. T. Martin Ma presented results of the SANDSTORM meta-analysis, which evaluated the sequencing of short-term ADT with radiotherapy in patients with non-metastatic prostate cancer.
Dr. Ma began his presentation by noting that the sequencing of systemic therapies with radiotherapy has been associated with differential survival benefits in multiple malignancies. The sequencing of ADT with RT (proportion of neoadjuvant versus adjuvant) has been variable across multiple trials:
- TROG and Canadian trials: favor prolonged neoadjuvant ADT
- NRG/RTOG trials: favor mixed neoadjuvant/concurrent ADT
- EORTC trials: favor concurrent/adjuvant ADT
As such, there has been limited data to directly compare ADT sequencing strategies. To date, only two randomized trials have ever compared ADT sequencing:
- RTOG 9413: 2x2 randomization of prostate-only radiotherapy (PORT) versus whole pelvis radiotherapy (WPRT); neoadjuvant/concurrent versus adjuvant ADT (total 4 months).
- Results of this trial suggested a significant interaction between radiotherapy field size and ADT sequencing1
- Ottawa 0101: Neoadjuvant (4 months) + concurrent (2 months) versus concurrent (2 months) + adjuvant (4 months). All patients received PORT only
- No statistically significant difference in biochemical recurrence-free survival between the two arms2
Pooled individual patient data (IPD) analysis of these two trials (RTOG 9413 and Ottawa 0101) of patients receiving PORT only (N=1,065), demonstrated more favorable outcomes in patients receiving adjuvant ADT (PFS, bRFS, distant metastasis, and metastasis-free survival), with no significant differences in prostate cancer-specific or overall mortality.
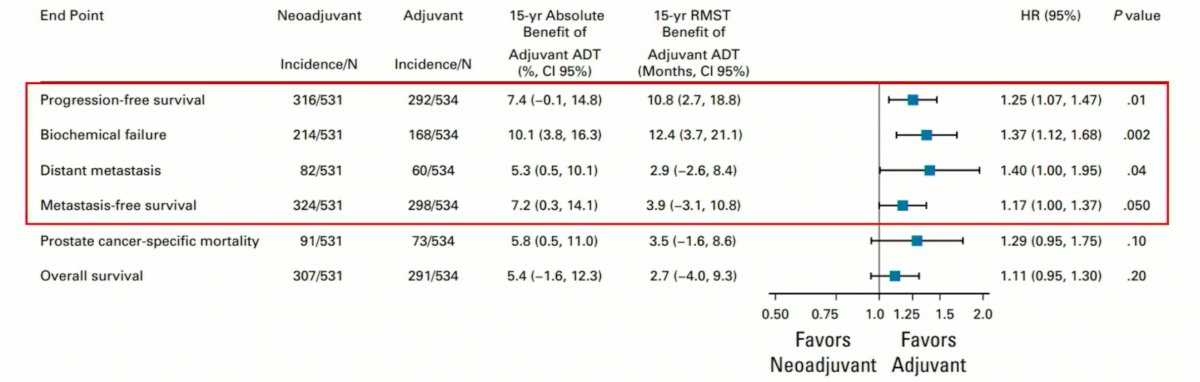
Similarly, the MARCAP IPD meta-analysis of 7 trials (3 with neoadjuvant ADT extension and 4 of adjuvant ADT prolongation) demonstrated no benefit in neoadjuvant ADT extension, but MFS and OS benefits with adjuvant ADT prolongation.3
The objective of the SANDSTROM meta-analysis was to investigate the impact of ADT sequencing for men receiving ADT for localized prostate cancer with PORT or WPRT. The primary endpoint was metastasis-free survival. IPD from 12 randomized trials from the MARCAP consortium that included patients receiving neoadjuvant/concurrent or concurrent/adjuvant short-term ADT (4-6 months) with RT were included (RTOG 8610, RTOG 9202, RTOG 9408, RTOG 9413, RTOG 9910, MRC RT01, PCS II, CHHiP, Ottawa 0101, DART 01/05 GICOR, EORTC 22961, EORTC 22991). This study included 7,409 patients of whom 6,325 received neoadjuvant/concurrent and 1,084 concurrent/adjuvant. The median follow-up was 10.2 years. Inverse probability of treatment weighting (IPTW) with propensity scores derived from age, initial PSA, Gleason score, T stage, RT dose and mid-trial enrollment year was performed. IPTW-adjusted Cox regression models and IPTW-adjusted Fine and Gray competing risk models were used in this setting. The restricted mean time lost was used as an alternative measure of the treatment effect.
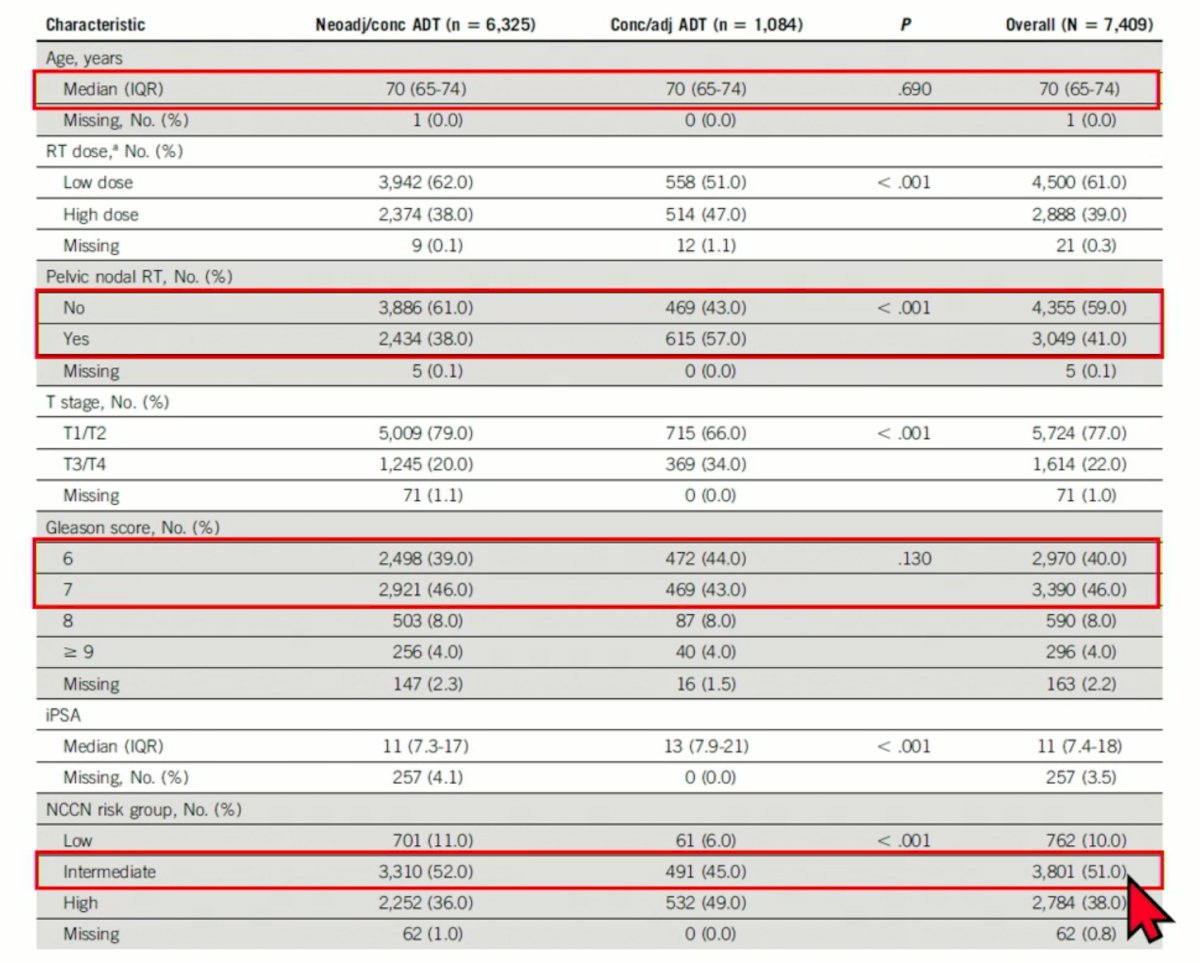
Dr. Ma next presented the baseline characteristics of the study cohort. He noted that the median age was 70 years, significantly more patients in the concurrent/adjuvant group received pelvic nodal RT (57% versus 38%, p<0.001), the majority of patients had a Gleason Score of 6-7, and approximately half were in the intermediate risk group.
There was a significant interaction between ADT timing and RT field size (PORT versus WPRT) for the outcomes of biochemical recurrence, distant metastases, prostate cancer-specific mortality, other-cause morality, metastasis-free survival (p<0.02 for all), but not overall survival (p=0.2) after IPTW adjustment. For subsequent analyses, the authors dichotomized the patients in two cohorts (PORT and WPRT) and performed independent analyses in each cohort per an a priori analysis plan.
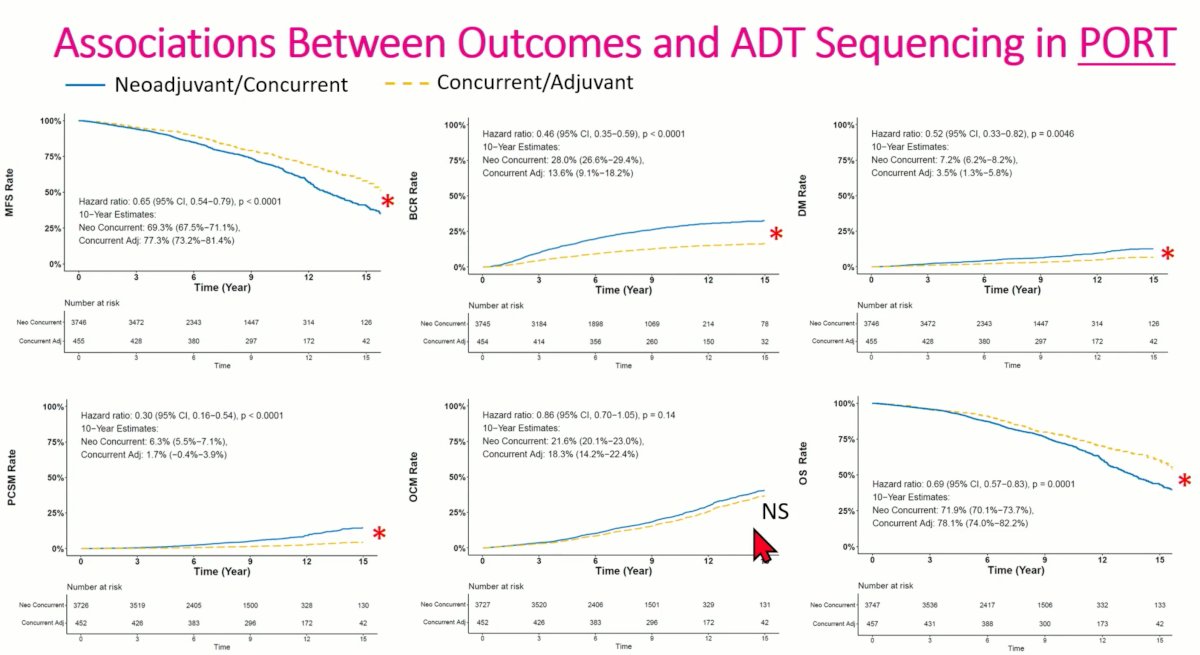
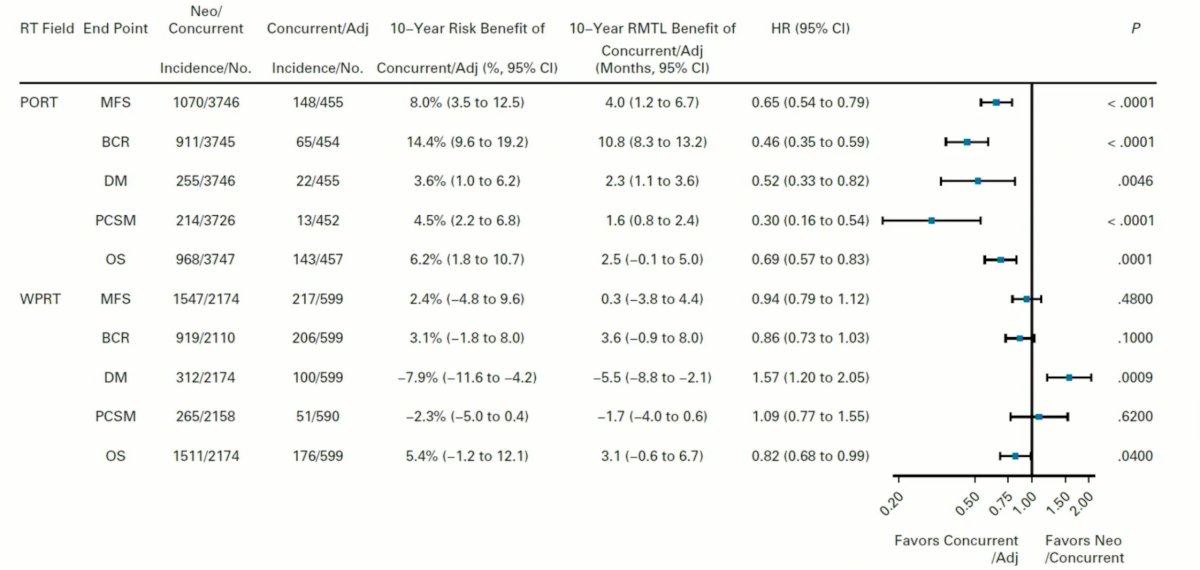
Dr. Ma first presented analysis for the patients receiving PORT only. Consistent results were seen in favor of concurrent/adjuvant ADT (yellow-dashed line) for MFS (HR: 0.65, 95% CI: 0.54 – 0.79, p<0.001), BCR (HR: 0.46, 95% CI: 0.35 – 0.59, p<0.001), DM (HR: 0.52, 95% CI: 0.33 – 0.82, p=0.0046), PCSM (HR: 0.30, 95% CI: 0.16 – 0.54, p<0.001), and OS (HR: 0.69, 95% CI: 0.57 – 0.83, p<0.001). Perhaps reassuringly, there were no significant differences in the other-cause mortality rates between patients receiving neoadjuvant/concurrent and adjuvant/concurrent ADT.
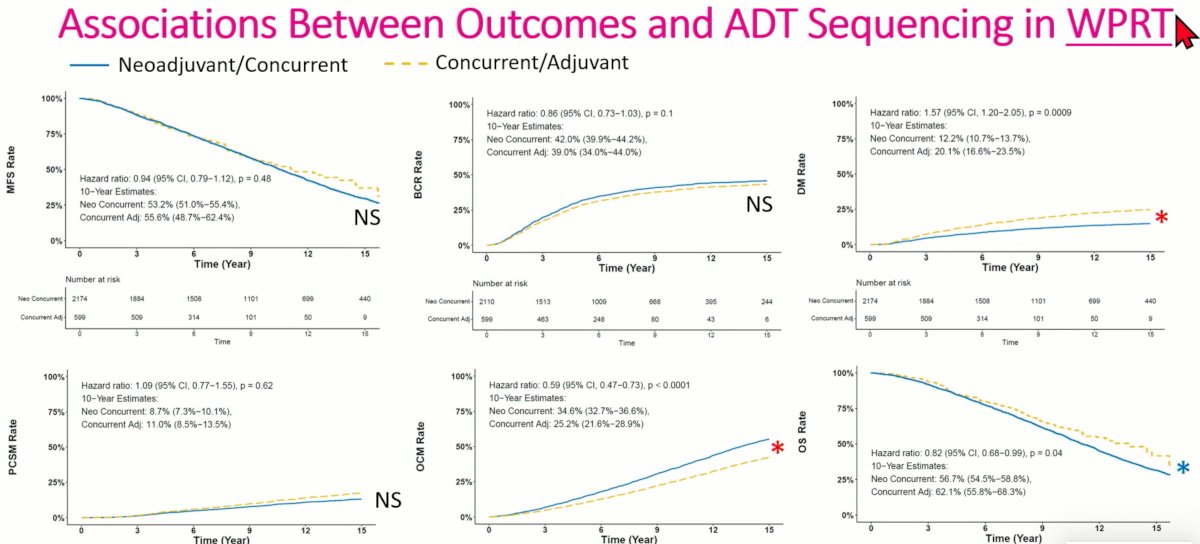
Conversely in patients receiving WPRT, benefit for neoadjuvant/concurrent ADT was noted for distant metastases (HR: 1.57, 95% CI: 1.20-2.05, p=0.0009), whereas superior other-cause (HR: 0.59, 95% CI: 0.47 – 0.73, p<0.0001) and overall mortality rates (HR: 0.82, 95% CI: 0.68 – 0.99, p=0.04) were seen in the concurrent/adjuvant arm.
Subgroup analysis summarizing the results from the above figures demonstrated consistent benefits in favor of concurrent/adjuvant ADT for all outcomes of interest in the PORT patients, whereas again lower distant metastases rates were noted in the neoadjuvant/concurrent arm and superior overall survival was noted in the concurrent/adjuvant arm in WPRT patients.
The authors concluded as follows:
- The impact of ADT sequencing on outcomes is dependent on the radiotherapy field size (PORT versus WPRT)
- When PORT is delivered, concurrent/adjuvant short-term ADT sequencing is associated with optimal oncologic outcomes, including overall survival
- These data strongly suggest that concurrent/adjuvant ADT should be the standard of care when short-term AD and PORT are indicated
- The effects are not as clear for patients receiving WPRT, though neoadjuvant/concurrent short-term ADT may be preferred given its distant metastasis benefit
Presented by: T. Martin Ma, MD, PhD, Resident Physician, Department of Radiation Oncology, University of California, Los Angeles, CA, USA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 American Society of Radiation Oncology (ASTRO) Annual Hybrid Meeting, San Antonio, TX, Sat, Oct 22 – Wed, Oct 26, 2022.
References:
- Roach, M et al. Sequence of hormonal therapy and radiotherapy field size in unfavourable, localised prostate cancer (NRG/RTOG 9413): long-term results of a randomised, phase 3 trial. Lancet Oncol. 2018;19(11):1504-1515.
- Malone, S et al., Sequencing of Androgen-Deprivation Therapy With External-Beam Radiotherapy in Localized Prostate Cancer: A Phase III Randomized Controlled Trial. J Clin Oncol. 2020; 20;38(6):593-601.
- Kishan, A et al., Androgen deprivation therapy use and duration with definitive radiotherapy for localised prostate cancer: an individual patient data meta-analysis. Lancet Oncol. 2022; 23(2):304-316


