(UroToday.com) The 2022 ASTRO annual meeting featured a prostate cancer session, including a presentation by Dr. Howard Sandler discussing challenges and solutions during the COVID pandemic for patient retention and physician engagement in the phase 3 ATLAS study of apalutamide added to ADT in high-risk localized or locally advanced prostate cancer. The phase 3 ATLAS study (NCT02531516) is investigating if treatment intensification with apalutamide to neoadjuvant and adjuvant treatment with gonadotropin-releasing hormone agonist (GnRHa) and external beam radiation therapy improves metastasis-free survival (MFS) in high-risk patients. To determine MFS improvement, long-term patient retention and physician engagement are critical. At ASTRO 2022, Dr. Sandler and colleagues described initiatives used for patient and physician participation, noting specific challenges related to patient retention during the COVID-19 pandemic, while maintaining trial integrity.
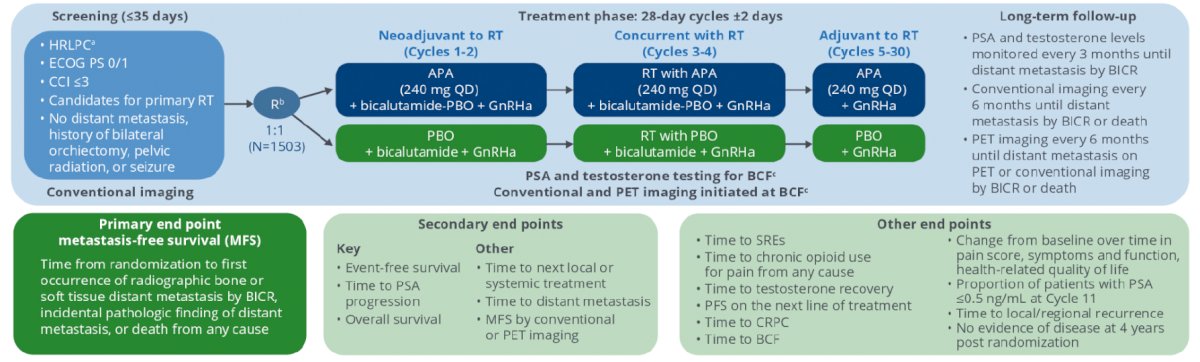
Eligible high-risk localized or locally advanced prostate cancer patients (Gleason score ≥ 8 or 7; if Gleason score 7, prostate-specific antigen ≥ 20 ng/mL; stage ≥ cT2c; ECOG PS 0/1; Charlson Comorbidity Index ≤ 3) stratified to Gleason score, pelvic nodal status, use of brachytherapy boost, and region were randomized 1:1 to apalutamide or placebo plus GnRHa for 30 (28 day) treatment cycles. Treatment was applied neoadjuvant/concurrent to external beam radiation therapy with apalutamide 240 mg/day vs bicalutamide 50 mg/d for 4 cycles; 26 cycles are completed adjuvantly post external beam radiation therapy with apalutamide 240 mg/day vs placebo. The ATLAS study design is as follows:
To maximize patient retention to MFS primary end point, especially considering impact of the COVID-19 pandemic, retention initiatives that can be chosen by sites per local regulation are:
- Reimbursing travel expenses
- Arranging travel for appointments
- Visiting nurse appointments for treatment management
- Using local labs and onsite radiology to reduce travel
- Using third-party vendors to maintain contact information
- Regular patient newsletters
To support consistent physician engagement, initiatives include materials on patient retention, site newsletters, teleconferences on reducing patient engagement barriers, and training on study updates.
Patients (n = 1503) were randomized at 266 sites in 24 countries (North America, Latin America, Europe, Asia), and the study is ongoing. Patient retention and physician engagement initiatives were incorporated at nearly all sites in all countries:
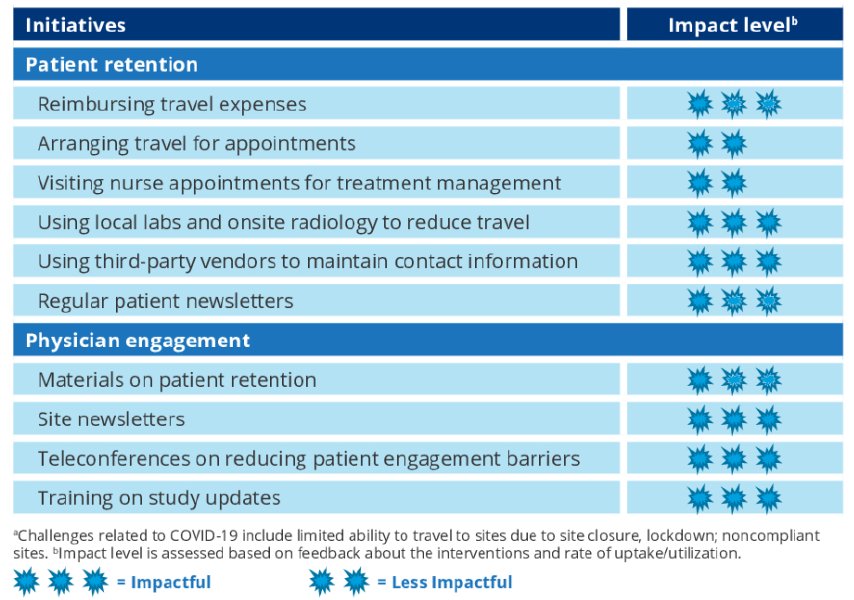
For patient retention, most sites (95%) are participating in travel reimbursement, coordination with local labs and onsite radiology, and patient newsletters; other program participation is varied. For physician engagement, all sites are receiving materials to retain patients and site newsletters. As of January 31, 2022, 96% of patients remaining eligible for follow-up have been retained on the study. With the retention initiatives implemented, the overall dropout rate can be maintained below the expected and statistically acceptable limits.
Dr. Sandler concluded his presentation discussing challenges and solutions during the COVID pandemic for patient retention and physician engagement in the phase 3 ATLAS study with the following concluding messages:
- Despite the challenges of the COVID-19 pandemic, very low dropout rates have been observed in the ATLAS trial
- These initiatives have resulted in high levels of patient retention and physician engagement post treatment and aid in understanding how to enhance long-term clinical trial outcomes
Presented by: Howard M. Sandler, MD, Cedars-Sinai Medical Center, Los Angeles, CA
Co-Authors: N. Shore2, D. Dearnaley3, S. J. Freedland1,4, M. R. Smith5, R. Rosales6, S. D. Brookman-May6,7, A. P. Dicker8, M. R. McKenzie9, A. Bossi10, A. Widmark11, T. Wiegel12, J. Martin13, B. Miladinovic14, F. Lefresne6, M. Ciprotti15, S. A. McCarthy16, S. D. Mundle16, B. F. Tombal17, and F. Y. Feng18; 1Cedars-Sinai Medical Center, Los Angeles, CA, 2Carolina Urologic Research Center, Myrtle Beach, SC, 3The Institute of Cancer Research and Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom, 4Durham VA Health Care System, Durham, NC, 5Massachusetts General Hospital Cancer Center, Boston, MA, 67Ludwig-Maximilians-University, Munich, Germany, 8Sidney Kimmel Cancer Center, Thomas Jefferson University, Philadelphia, PA, 9British Columbia Cancer Agency, Vancouver, BC, Canada, 10Institute Gustave Roussy, Villejuif, France, 11Department of Radiation Sciences, Oncology, Umeå University, Umeå, Sweden, 12University Hospital Ulm, Ulm, Germany, 1314151617Cliniques Universitaires Saint-Luc, Brussels, Belgium, 18Helen Diller Family Comprehensive Cancer Center, University of California San Francisco, San Francisco, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Radiation Oncology (ASTRO) Annual Hybrid Meeting, San Antonio, TX, Sat, Oct 22 – Wed, Oct 26, 2022.


