Dr. Lara began by emphasizing three main principles in the treatment of mRCC:
- The goal is cure, or the prolongation of life.
- Immunotherapy offers the best chance of cure.
- Angiogenesis is active throughout the natural history of clear cell RCC.
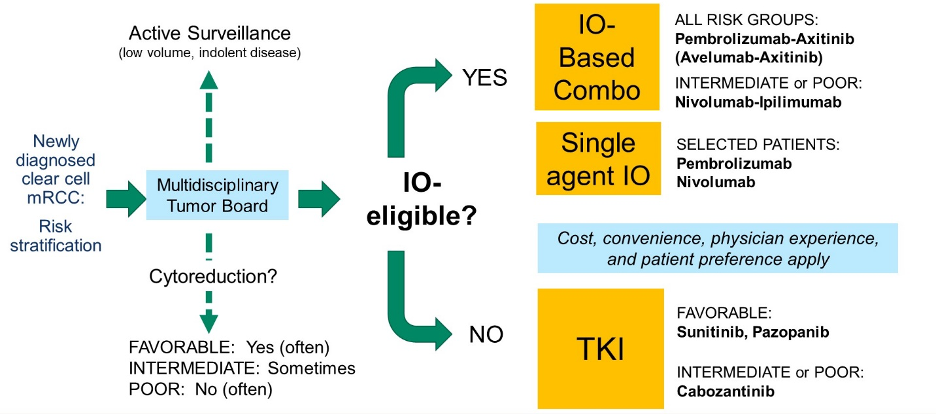
Among patients with mRCC, Dr. Lara highlighted a decision tree driven by multi-disciplinary tumor board assessment of the patient’s risk stratification, suitability for active surveillance, and the role of cytoreduction. When assessing systemic therapy options, the initial decision is driven by eligibility for immunotherapy with checkpoint inhibitors, with stratification according to risk category.
Dr. Lara then highlighted the prognostic value of the Heng criteria (IMDC risk strata) among patients who received VEGF-targeted therapy. Notably, patients with favourable risk disease had median overall survival of 37 months, those with intermediate-risk disease of 28 months, and those with poor-risk disease of 9.4 months.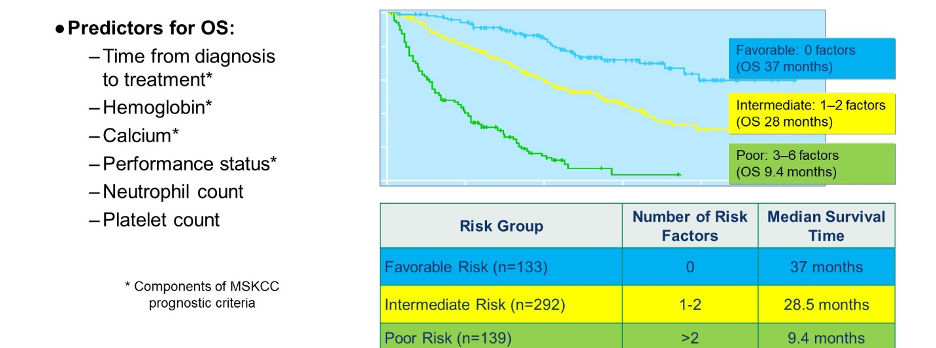 Dr. Lara then reviewed each of the steps highlighted in the initial decision treat.
Dr. Lara then reviewed each of the steps highlighted in the initial decision treat.
He began by examining patients with mRCC who are candidates for active surveillance. He highlighted phase II data from Dr. Rini of a surveillance period among 22 patients with asymptomatic mRCC. Median time to treatment initiation (as a result of symptomatic progression) was 14.9 months.
Moving to the question of cytoreductive nephrectomy, he first presented data from the CARMENA trial which demonstrated that sunitinib alone was not inferior to sunitinib following cytoreductive nephrectomy among patients with intermediate or poor-risk mRCC. However, in his opinion, cytoreductive nephrectomy candidacy should be a decision driven according to individualized risk with input of multi-disciplinary tumor boards. Dr. Lara suggested that most patients with favorable disease and some with intermediate-risk disease remain candidates for cytoreductive nephrectomy. Additionally, those large or symptomatic tumors and a low volume of metastatic disease remain preferential candidates for cytoreductive nephrectomy. In contrast, many patients with intermediate-risk disease and nearly all with poor-risk disease should be prioritized for systemic therapy first.
Metastatasectomy in mRCC may also be considered. However, Dr. Lara cautioned that this should be reserved for highly selected patients as the quality of evidence to support this approach is limited. Patients most likely to benefit include those with good performance status, isolated or oligometastatic disease, a disease-free interval following nephrectomy of 2 years or more, the absence of lymph node involvement, and lung-only disease.
When considering systemic therapy, Dr. Lara highlighted a variety of reasons one might consider monotherapy including the simplicity of the treatment regime, lower cost, tolerability, adherence, and reduced drug-drug interactions.
 Historically, such a monotherapy approach has been the standard of care with single-agent angiogenesis inhibitors, mTOR inhibitors, and immunotherapy being commonly used. The combination of bevacizumab and interferon was the only FDA-approved combination approach until recently.
Historically, such a monotherapy approach has been the standard of care with single-agent angiogenesis inhibitors, mTOR inhibitors, and immunotherapy being commonly used. The combination of bevacizumab and interferon was the only FDA-approved combination approach until recently.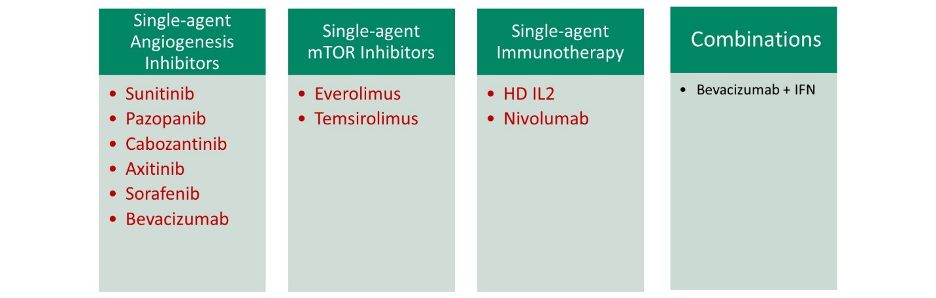
However, in the past few years, a number of combination approaches have now been demonstrated to be beneficial, the majority of which are now FDA-approved: nivolumab + ipilimumab, pembrolizumab + axitinib, avelumab + axitinib, lenvantinib + everolimus, and cabozantinib + nivolumab. Many of these have become standard of care.
Dr. Lara concluded that the standard of care for first-line therapy in patients with mRCC in 2020 centers on immunotherapy-based combination therapy and most patients should be considered for this approach. Both immunotherapy-TKI combinations (pembrolizumab-axitinib in all risk groups; avelumab-axitinib in all risk groups; and pending results for nivolumab-cabozantinib), as well as immunotherapy doublet therapy (nivolumab-ipilimumab in intermediate and poor-risk patients), have level I evidence to support their use.
However, it’s important to consider those who are not eligible for immunotherapy, including patients with active autoimmune disease, a history of solid organ transplantation, those requiring supraphysiologic corticosteroids, those on chronic immunosuppressive therapy, and according to specific patient preferences (for example, those who refuse intravenous agents).
Conversely, Dr. Lara highlighted patients who could be considered for monotherapy with checkpoint inhibitors. While few patients fit this category, such an approach could be considered for patients who are ineligible or refuse a VEGFR-TKI containing combination or who are averse to ipilimumab. Preliminary data on this approach suggest objective response rates of 34-36% and progression-free survival of 7-8 months.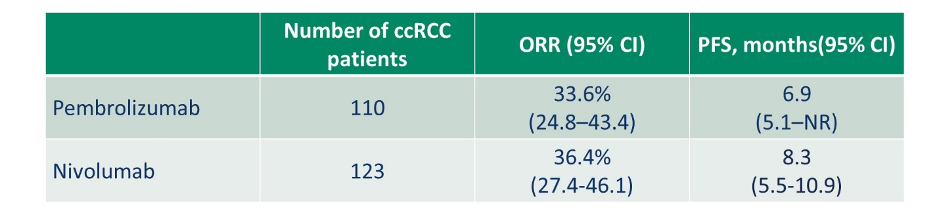 Dr. Lara then addressed the question of high dose-IL2 monotherapy. While this was a standard approach prior to the introduction of TKIs, this is still listed as a monotherapy option in some guidelines. Dr. Lara suggested that almost no patients should be considered for this approach but, when considered, it should be reserved for robust patients with excellent performance status and normal end-organ function due to the potential for long term survival. However, it should be noted that such an approach requires inpatient care and high toxicity.
Dr. Lara then addressed the question of high dose-IL2 monotherapy. While this was a standard approach prior to the introduction of TKIs, this is still listed as a monotherapy option in some guidelines. Dr. Lara suggested that almost no patients should be considered for this approach but, when considered, it should be reserved for robust patients with excellent performance status and normal end-organ function due to the potential for long term survival. However, it should be noted that such an approach requires inpatient care and high toxicity.
Further, while mTOR monotherapy (with temsirolimus) remains FDA-approved for the frontline treatment of mRCC, Dr. Lara suggested that there is little justification for its use in the first-line setting.
Finally, Dr. Lara considered the question of when to offer VEGFR-TKI monotherapy. He highlighted four circumstances: those ineligible for immunotherapy, those who refuse immunotherapy, those who are intolerant of immunotherapy, and in selected subsets of patients. These subsets include those with bone-only disease, those with non-clear cell histology, and some favorable-risk patients. In favorable-risk disease, the CheckMate214 trial demonstrated high response rates and progression-free survival for patients receiving sunitinib, as compared to nivolumab and ipilimumab. 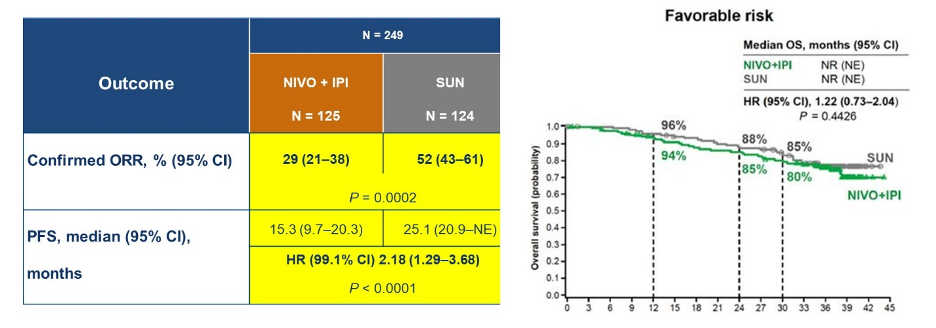 Similarly, in the KEYNOTE-426 trial, there was no overall survival benefit or progression-free survival benefit for the combination of pembrolizumab and axitinib as compared to sunitinib in the favorable risk subgroup.
Similarly, in the KEYNOTE-426 trial, there was no overall survival benefit or progression-free survival benefit for the combination of pembrolizumab and axitinib as compared to sunitinib in the favorable risk subgroup.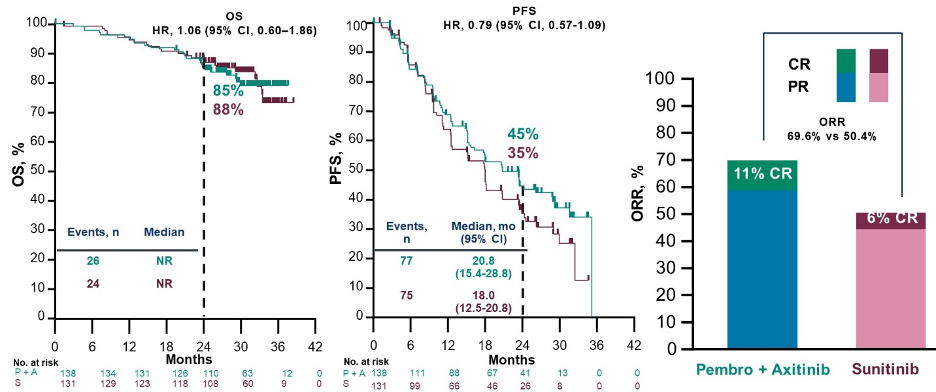
In summary, Dr. Lara highlighted the importance of risk stratification, multi-disciplinary input, and assessment of immunotherapy eligibility prior to decision making. For most patients, combination immunotherapy-based therapy should be considered standard of care with monotherapy limited to a small and diminishing, subset.
Presented by: Primo Lara, MD, UC Davis Comprehensive Cancer Center
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, @WallisCJD on Twitter at the ASCO20 Virtual Education Program, #ASCO20, August 8-10, 2020


