(UroToday.com) The 2022 GU ASCO Annual meeting included a prostate cancer session highlighting work from Dr. Zachery R. Reichert and colleagues presenting results of the TRAP trial, targeting resistant prostate cancer, with or without DNA repair defects, using the combination of ceralasertib and olaparib. Men with metastatic, castration resistant prostate cancer (mCRPC) harboring DNA repair defects (~20%) achieve a radiographic progression free survival of 7.4 months with PARP inhibitors. Preclinical studies combining PARP inhibitors (olaparib) and DNA damage checkpoint inhibitor (ATR inhibitor, ceralasertib) show synergy, providing the rationale to test this combination in men with mCRPC, including where single agent olaparib has been shown to be active.
Two cohorts were accrued to a trial combining ceralasertib with olaparib in men with or without DNA repair defects. All patients progressed on ≥1 prior mCRPC therapy with no prior PARP inhibitors or platinum chemotherapy. The primary endpoint was disease response (confirmed PSA decline ≥50% and/or RECIST response), while disease progression was defined per Prostate Cancer Working Group 3 definition. Each cohort was analyzed independently for disease endpoints, while both groups were combined for toxicity assessments.
The 12 person DNA repair-deficient cohort allowed patients with germline BRCA2 loss (n = 4), somatic BRCA2 loss (n = 1) and ATM loss (n = 1 germline, n = 5 somatic and n = 1 somatic with unknown germline). There were 35 men without BRCA2/BRCA1 or ATM genomic loss that was accrued to the DNA repair-proficient cohort. A summary of the baseline characteristics is as follows:

These men had next-generation sequencing (NGS) on contemporary biopsies (prior to enrolment without intervening therapy, n = 12), prior NGS on metastatic tissue (n = 10), prior NGS on primary prostatic tissue (n = 3), or cell-free analyses (n = 5). In the most recent follow-up presented at GU ASCO 2022, 4 of the 12 DNA repair-deficient patients have responded and 4 of 35 DNA repair-proficient patients have responded:
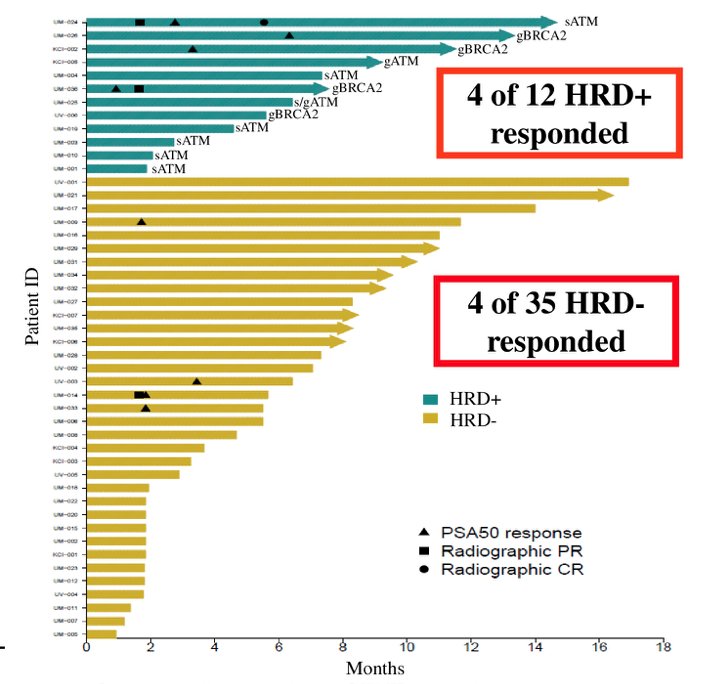
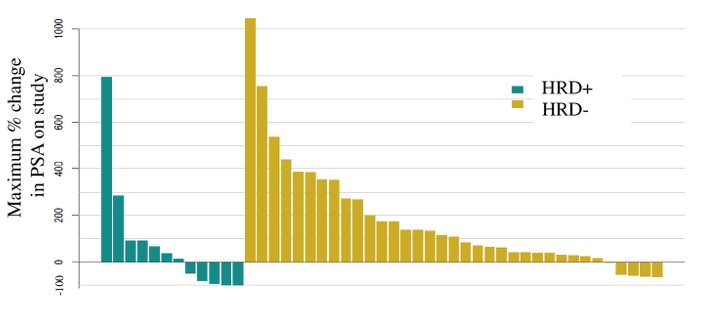
As follows are the results of the PSA waterfall plot:
With regards to safety, 77% of patients had any grade treatment-related adverse events, including 21% of patients that had a grade 3 event, with no reported grade 4 or 5 adverse events.
Dr. Reichert concluded his presentation of the TRAP trial with the following take-home messages:
- DNA repair-proficient patients had a response rate of only 11%, but several patients experienced prolonged tumor control; 37% of patients had disease control >7 months, which is approximately the same as the rPFS seen with olaparib in HRD+ patients in the PROfound trial [1]
- DNA repair-deficient patients with BRCA2 loss had a good response (3 of 4 patients) and all were still on therapy. Patients with ATM loss had a poor response (1 of 8 patients) and two were still on therapy
- There were no unexpected treatment related adverse events observed
Clinical trial information: NCT03787680.
Presented by: Zachery R. Reichert, MD, PhD, University of Michigan, Ann Arbor, MI
Co-Authors: Michael Edward Devitt, Joshi J. Alumkal, David C. Smith, Megan Veresh Caram, Philip Palmbos, Ulka N. Vaishampayan, Ajjai Shivaram Alva, Thomas Braun, Sarah Elizabeth Yentz, Phoebe A. Tsao, Robert Dreicer, Frank Cameron Cackowski, Neel Shah, Emma Dean, Simon Smith, Elisabeth I. Heath
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References: