(UroToday.com) The 2022 GU ASCO Annual meeting included a urothelial carcinoma session featuring work from Dr. Tibor Csoszi and colleagues presenting results assessing clinical parameters associated with efficacy in the phase 2 KEYNOTE-052 and phase 3 KEYNOTE-361 trials of first-line pembrolizumab in advanced disease. First-line treatment with pembrolizumab monotherapy has shown durable clinical activity in selected patients with advanced/unresectable or metastatic urothelial carcinoma. Cisplatin-ineligible patients with advanced urothelial carcinoma who were not previously treated with chemotherapy were enrolled in KEYNOTE-052,1 and patients with untreated advanced urothelial carcinoma were enrolled in KEYNOTE-361.2 In a pooled population of patients with advanced urothelial carcinoma from the single-arm phase 2 KEYNOTE-052 and the randomized, open-label, phase 3 KEYNOTE-361 studies, this exploratory analysis evaluated the relationship between baseline characteristics and clinical outcomes of first-line pembrolizumab monotherapy. The study designs for these two trials are as follows:

For analysis of predictive factors for ORR and OS in pembrolizumab-treated patients, the purposeful selection method was used to build the multivariable logistic regression model (ORR) and multivariable Cox model (OS), beginning with a univariable analysis of each independent variable. Any variable in the univariate model with P < 0.10 was a candidate for the multivariate model. The stepwise selection method was used to select the variables in the final model, with significance of the final model set at P < 0.05. Data cutoff dates were September 26, 2020 (KEYNOTE-052) and April 29, 2020 (KEYNOTE-361).
This pooled analysis included 681 patients treated with pembrolizumab monotherapy (KEYNOTE-052, N = 374; KEYNOTE-361, N = 307; 170 patients were cisplatin ineligible). Over a median follow-up of 51.9 months (range, 22.0-65.3), the ORR was 29.4% (95% CI, 26.0-32.9; 69 CRs, 131 PRs) and median DOR was 33.2 months (range, 1.4+ to 60.7+). By multivariate analysis, independent factors significantly associated with higher ORR were PD-L1 status (CPS ≥10 vs CPS < 10; OR 1.90, 95% CI 1.33-2.71), site of metastasis (lymph node only vs visceral disease; OR 1.66, 95% CI, 1.06-2.59), liver involvement (absent vs present; OR 1.75, 95% CI 1.06-2.89), and baseline hemoglobin level (≥10 vs < 10 g/dL; OR 2.17, 95% CI 1.09-4.31):

By multivariate analysis, there were several independent factors significantly associated with improved OS as highlighted in the following figure:
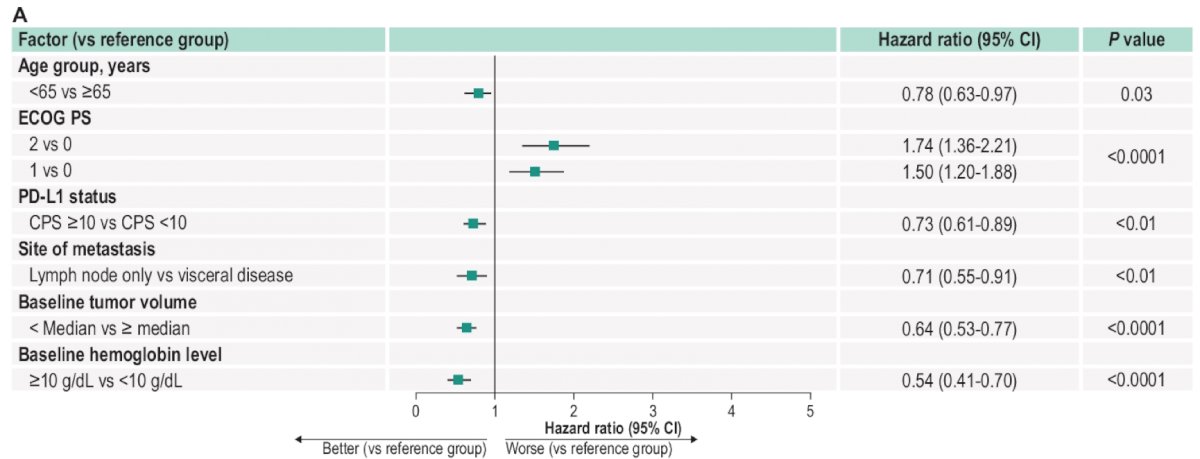
In the pooled population, the median overall survival was 12.5 months (95% CI 11.2-14.6) with a 24-month OS rate of 34.2%:

Dr. Csoszi concluded this presentation of the pooled analysis of KEYNOTE-052 and KEYNOTE-361 with the following summary points:
- This exploratory multivariate analysis identified numerous factors, including PD-L1–positive status (CPS ≥10), lymph node only metastasis, and lower ECOG PS score, associated with improved clinical outcomes in patients with advanced urothelial carcinoma treated with first-line pembrolizumab monotherapy
- These factors may be important to consider in the design of future studies with pembrolizumab
Co-Authors: Thomas Powles, Ajjai Shivaram Alva, Daniel E. Castellano, Mustafa Ozguroglu, Peter H. O'Donnell, Yohann Loriot, Noah M. Hahn, Aude Flechon, Alejo Rodriguez-Vida, Ronald De Wit, Susanna Y. Cheng, Stephane Oudard, Christof Vulsteke, Evan Y. Yu, Jianxin Lin, Kentaro Imai, Blanca Homet Moreno, Arjun Balar, Petros Grivas
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022
References:
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483-1492.
- Powles T, Csoszi T, Ozguroglu M, et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomized, open-label, phase 3 trial. Lancet Oncol. 2021 May 26;S1470-2045(21)00152-2.