Scott Williams, MD presented a randomized trial comparing 18Flourocholine-PET/CT (FCH) to computed tomography of the abdomen and pelvis plus 99mTc-whole body bone scan (which is regarded as the conventional imaging) to determine imaging performance in prostate cancer (PC). The rationale for this study was driven by the allure of increased sensitivity off FCH. The authors of this study wanted to answer the questions: Can more staging information can be captured? Can increased sensitivity translate into management changes? And finally, can FCH-PET/CT be a first line imaging “one-stop shop”?
This was a prospective single-center two-arm 1:1 randomized trial that enrolled men with newly diagnosed or biochemically recurrent PC to first-line imaging with either conventional imaging (CT abdomen and pelvis, and whole body bone scan) or FCH (Australian New Zealand Clinical Trials Registry ACTRN12608000641392). Exclusion criteria included patients who had imaging for staging within 8weeks prior to recruitment, androgen deprivation therapy (ADT) commenced within 6 months, any other malignancy within 5 years, and a life expectancy of less than 5 years. Patients without evidence of metastases proceeded to second-line imaging using the alternative imaging strategy. According to Dr. Williams, the primary aim was to determine whether FCH was more effective as a first-line imaging approach and resulting in a management change. The secondary endpoints included the incremental utility of second-line imaging, any management impact, and negative predictive value based on progression-free survival (PFS). The study scheme is shown in Figure 1.
Figure 1 – Trial scheme:
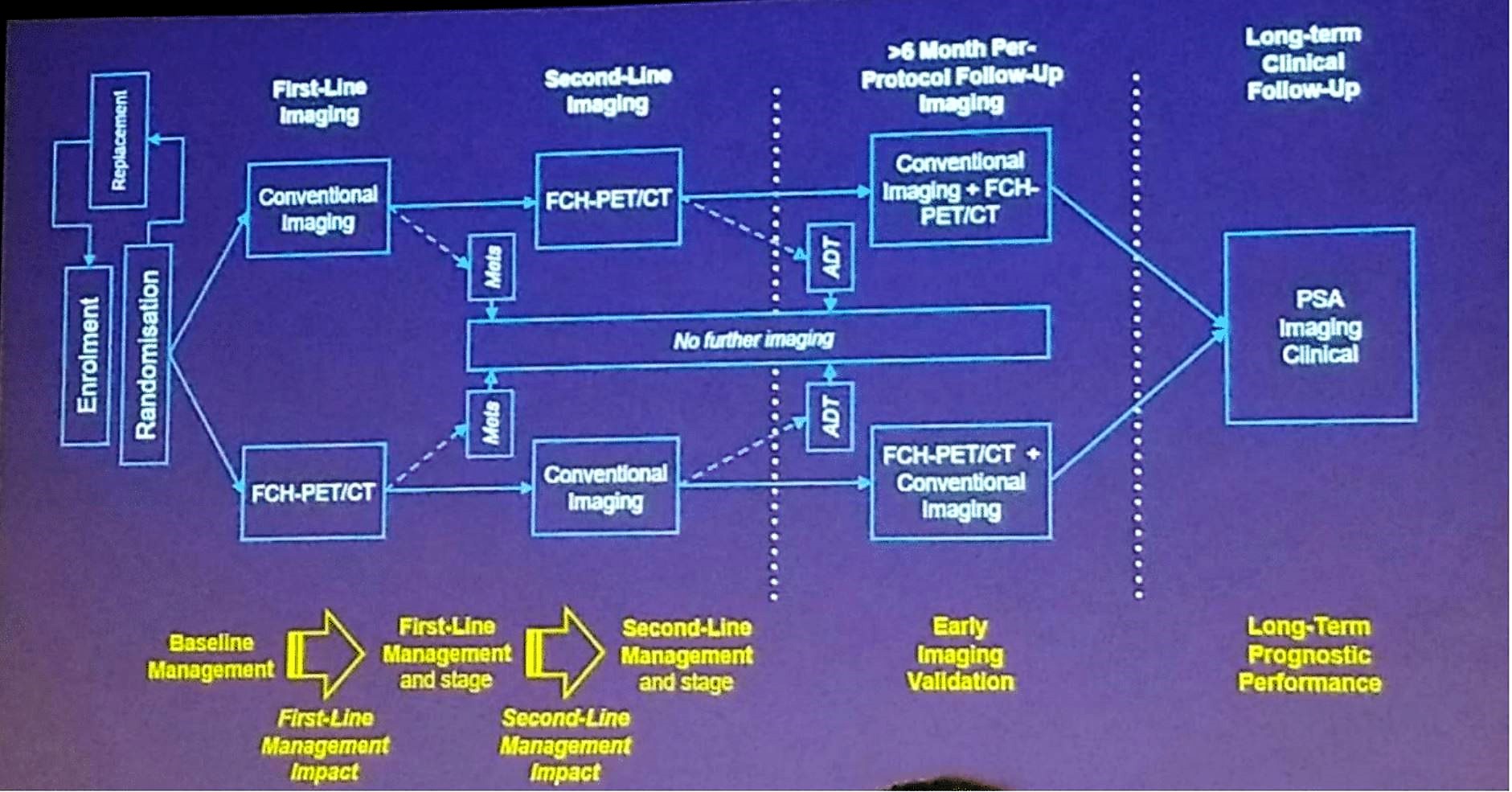
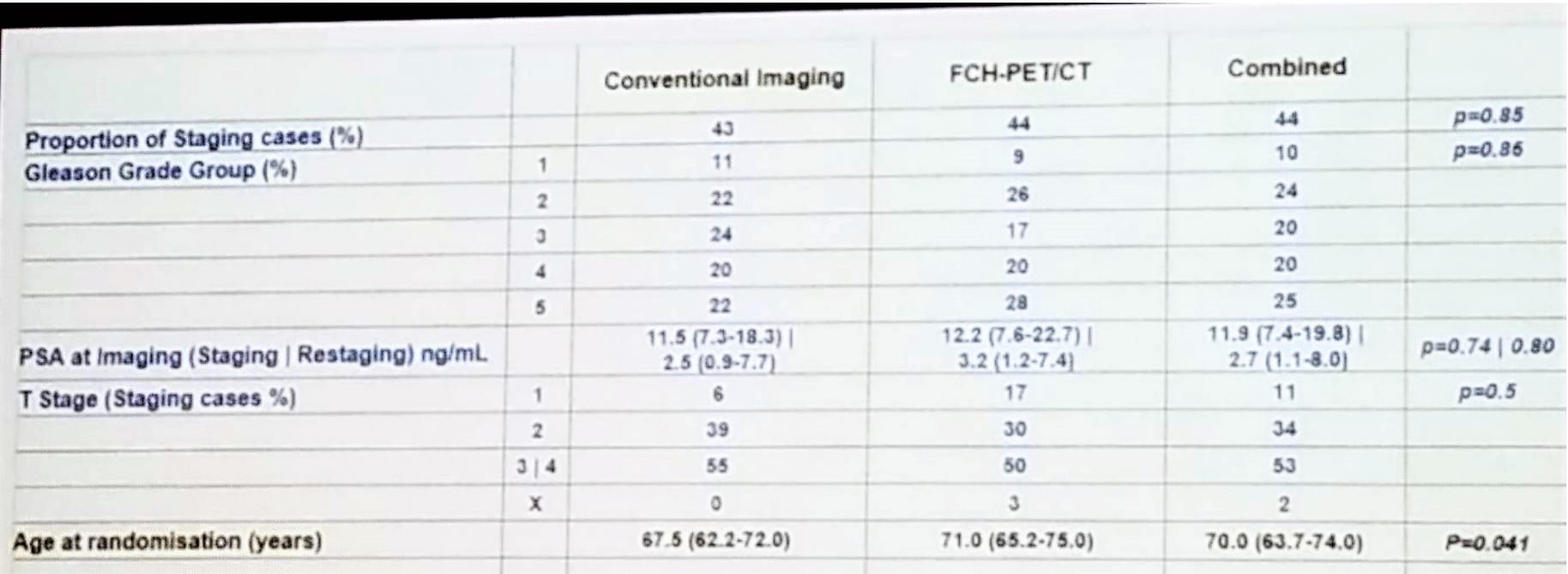
According to the results, 119 men were enrolled, but only 108 were deemed to be evaluable cases. Per protocol, follow-up imaging occurred in 102 men with 70 patients being imaged. The median follow-up was 46.6 months with 82 patients had undergone further imaging. A total of 63 patients had a clinical or radiological progression, and a total of 13 deaths had occurred, 5 of them resulting from prostate cancer. The demographic details of the trial are shown in Table 1.
Table 1 – Demographic data:
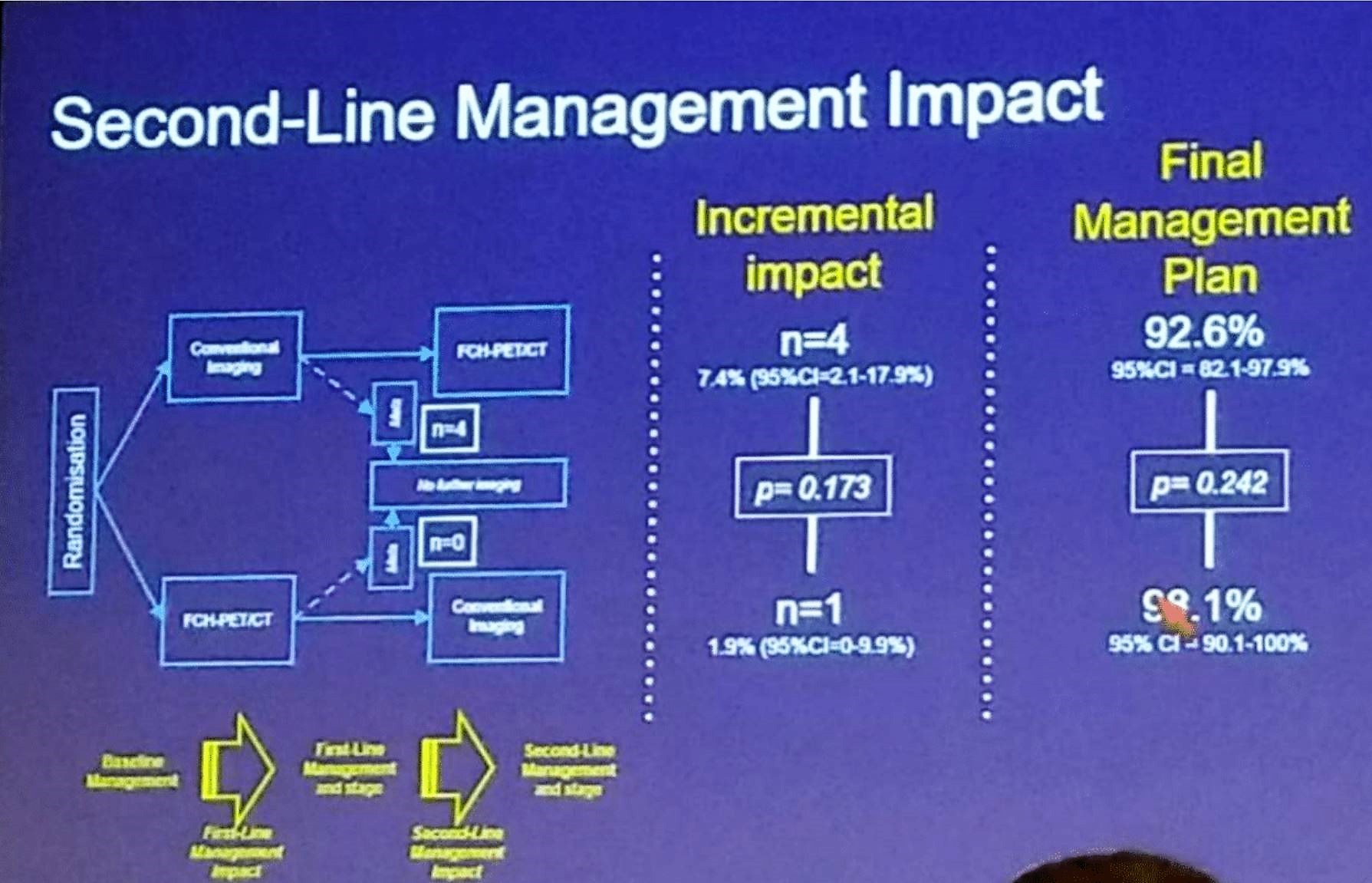
The first-and second-line management impact is summarized nicely in Figure 2, and figure 3. Overall, 70 men were eligible and undertook per protocol repeat imaging, with eight additional metastatic cases being identified. The overall rate of subsequent failure was not substantially different between the modalities.
Figure 2 –First line management impact:
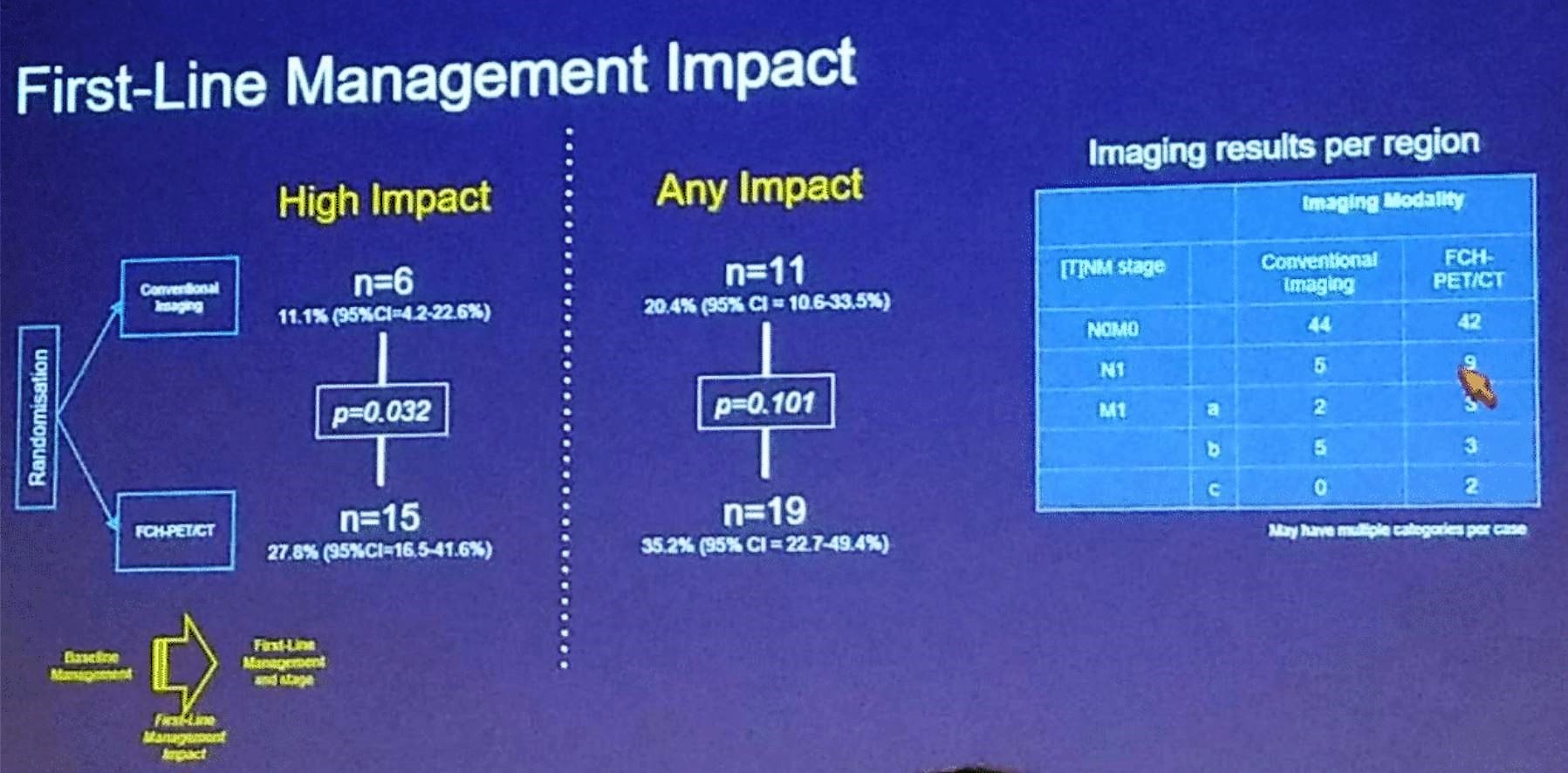
Figure 3 –Second line management impact:
Concluding his presentation, Dr. Williams stated that FCH-PET/CT offers more clinical utility as a first-line imaging modality than CT scan and whole-body bone scan. FCH appears to be an adequate replacement of conventional imaging as a solitary aging modality. FCH-PET/CT reduces the rate of equivocal findings, both in comparison to or before conventional imaging. Both approaches were shown to harbor a low negative predictive value and modest discrimination of outcomes. FCH-PET/CT may identify nodal metastases earlier without clear outcome differences. Management impact change was not associated with improved prognostic performance. Lastly, Dr. Williams believes that subsequent studies should focus on more selected cohorts and more robust endpoints.
Presented by: Scott Williams, MD, Peter MacCallum Cancer Centre, Melbourne, Australia
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA


