As highlighted by Dr. Nichols previously, the vast majority of the major breakthroughs in testicular management occurred in the 1960’s-1980’s –while there has continued to progress since then, it has been much smaller incrementally. Indeed, there hasn’t been any major breakthroughs in testicular cancer management in a few decades –most development has been fine-tuning. In Dr. Hamilton’s words, “we’re due!”
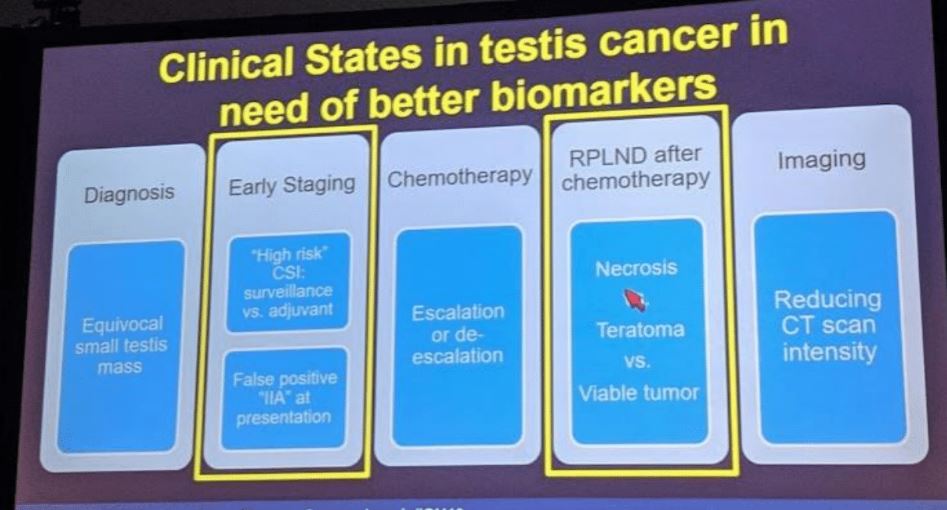
There are numerous clinical states in testicular cancer that could benefit from a new biomarker, and below they are nicely laid out –with an emphasis on two areas that are best to start with and are the lowest hanging fruit in terms of short-term outcome data:
Micro RNA’s are small non-coding (~22 nucleotides)RNA segments that are not translated into proteins –but rather than can interact with mRNA and impact post-translational expression –functionally serving an oncogenes or tumor suppressors.
The miRNA story in testicular cancer actually started in 2006 –Voorhoeve et al (Cell 2006) first reported on miRNA-372 and miRNA-373. By 2010, it had been identified that 4 miRNA’s (miRNA-371-373 and miRNA-302 clusters) were overexpressed in testicular cancer regardless of tumor type (seminoma or NSGCT), NSGCT subtype, or anatomical site (gonadal or extragonadal), hinting at the specificity of these molecules. However, this was all in primary tissue. Work over the next few years identified in serum and demonstrated that it has very fast half-lives (~3-7 hours, contrasted with the 5-7 day half-life of AFP!).
Work from a few groups, including Leao et al. (JUrol 2018) and Dieckmann et al. (EU 2017), demonstrated that while there were 4 specific miRNA’s that could be used as biomarkers, miRNA-371-3p individually outperformed the group as a whole. As such, the focus will be on this individual miRNA for the rest of the talk.
Retrospective data:
All the studies are limited by small sample sizes.
- Van Agthoven et al. (Oncotarget 2017) – compared the utility of miRNA-371 as a diagnostic test, using 250 patients with GCT, 104 controls, and 60 non-GCT cases. They found it had a 90% sensitivity for GCT, 86% specificity, and AUC of 0.95
- Dieckmann et al. (EU 2017) – miRNA-371 measured in 166 consecutive GCTs and 106 controls. They found expression correlated with tumor size and clinical stage. This suggested in may have quantitative benefit for monitoring therapy rather than a dichotomous (yes/no) use. It was also demonstrated to decrease with chemotherapy administration – but unfortunately, it was not correlated to post-chemotherapy outcomes.
- Mego et al. (J Cell Mol Med 2019) – in 180 patients starting first-line chemotherapy, miRNA-371 was associated with PFS and OS. But, importantly, on MV analysis, once risk category was accounted for, it was not clinically significant. Therefore, it needs to be assessed relative to known clinical predictors.
- Leao et al. (J Urol 2018) – work by Dr. Hamilton’s group in Toronto assessed 82 patients undergoing orchiectomy, chemotherapy and PCRPLND had serial miRNA 371 assessed. They found that:
- miRNA was a good predictor of chemotherapy response
- It was strongly associated with the finding of viable disease at the time of PCRPLND
- However, it was NOT elevated with the finding of teratoma – so it cannot distinguish between teratoma and fibrosis
- Nappi et al. (GU ASCO 2018) – in 41 men with CS1 GCT, 35 developed metastases. 24 of these patients had PCRPLND. They found that miRNA-371 had an AUC 0.98 for identifying the viable disease.
So miRNA-371 has all the features of an ideal biomarker! But there are limitations:
- No agreed upon standard methodology for measuring it
- No agreed upon cutoff for “normal” – studies report a relative ratio compared to so-called normal
- The teratoma issue – difficult clinical situation since it doesn’t identify teratoma, but this still requires treatment
Overcoming the teratoma issue – in a study by Shen et al. (Cell Reports 2018), they identified 137 patients with primary testicular cancer through TCGA. On tissue-based (not serum) sequencing, one thing they assessed was miRNA levels. Interestingly, while they confirmed that miRNA-371 was NOT associated with teratoma, miRNA-375 was elevated in teratoma. So, this may represent an opportunity for combining two biomarkers for an easier to use clinical test!
He lastly highlighted two prospective studies ongoing that will help add more knowledge to the field:
1) SWOG S1823

This trial is expected to open later this year
2) Children’s Oncology Group AGCT1531

This is not just for testicular cancer, but also for ovarian and EGCT cancers. This trial is already open.
Presented by: Robert J. Hamilton, MD, MPH, Princess Margaret Cancer Centre, University Health Network, Toronto, Canada
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @JEFFUrology) at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA