For this trial, eligibility criteria included histologically confirmed metastatic or recurrent renal cell carcinoma with sarcomatoid component of 25% or more on resected kidney tissue or exclusive sarcomatoid carcinoma on needle biopsy, ECOG performance status 0-2, measurable lesion by RECIST v1.1, and adequate cardiac, hepatic, renal and bone marrow function. Exclusion criteria included patients with uncontrolled hypertension, and/or prior exposure to gemcitabine or VEGFR TKI. Patients received gemcitabine 1,000 mg/m2intravenously on days 1 and 8 by 3-week cycle and axitinib 5 mg twice daily. The primary endpoint was objective response rate according to RECIST v1.1, and secondary end points included progression-free survival (PFS), OS, and treatment toxicity.
Between October 2014 and August 2018, 25 patients were enrolled in this clinical trial. The median age was 61years (range 33-80), and 84% of patients were male. ECOG performance status was 1 for 92% of patients, and 52% had prior radical nephrectomy. Clear cell carcinoma was the most common histology of carcinoma component, and median percentage of sarcomatoid component was 90% (range 25-100%). According to the IMDC risk stratification, there were 28% intermediate and 72% poor risk patients. Gemcitabine and axitinib were first line therapy for 88% of patients in the trial. The median number of gemcitabine-axitinib cycles was 6:56% achieved partial response, 28% stable disease, and 12%progressed on treatment. The objective response rate was 56% and the median duration of response was 2.5 months. With a median follow-up duration of 24.8 months, the median PFS was 4.2months (95%CI 2.3-6.1), and median OS was 8.4 months (95%CI 3.3-13.4months).
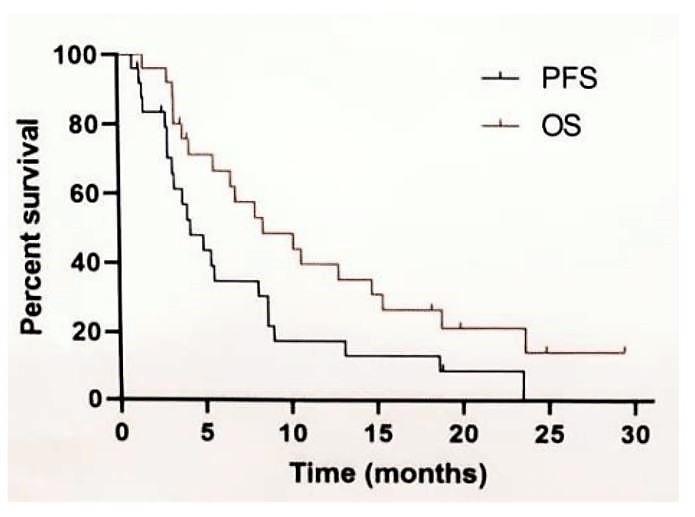
Despite gemcitabine and axitinib in combination demonstrating a degree of efficacy for patients with sarcomatoid renal cell carcinoma, the OS is still dismal at <9 months. Gemcitabine and axitinib may be considered a treatment option for patients with sarcomatoid renal cell carcinoma, however further larger studies are needed to confirm these findings, as well as a continued search for additional therapeutic options.
Presented by: Inkeun Park, Gachon University Gil Medical Center, Incheon, Korea
Written By: Zachary Klaassen, MD, MSc –Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter:@zklaassen_md at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
References:
1. Golshayan AR, George S, Heng DY, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol 2009 Jan 10;27(2):235-241.


