(UroToday.com) The 2022 ASCO annual meeting included a poster discussion session for kidney and bladder cancer, including a discussant presentation from Dr. Cristina Suarez discussing sequencing challenges in renal cell carcinoma (RCC). The abstracts discussed by Dr. Suarez included “Adjuvant pembrolizumab for post-nephrectomy RCC: Expanded efficacy analyses from KEYNOTE-564” presented by Dr. Toni Choueiri, “Pembrolizumab + axitinib versus sunitinib as first-line therapy for advanced clear cell RCC: Analysis of progression after first subsequent therapy in KEYNOTE-426” presented by Dr. Tom Powles, and “Impact of subsequent therapies in patients with advanced RCC receiving lenvatinib + pembrolizumab or sunitinib in the CLEAR study” presented by Dr. Martin Voss.
Dr. Suarez started by highlighting that the definition of PFS2 is the time from randomization to disease progression on next line of treatment, or death from any cause (whichever occurs first). Of note, this includes time periods that patients are on second line therapy:

The KEYNOTE-564 presentation focused on additional efficacy analyses of time to first subsequent drug treatment or any-cause death (TFST) and time from randomization to progression on next line of therapy or any-cause death (PFS2). The median time from randomization to the data cutoff date (June 14, 2021) was 30.1 months (range, 20.8-47.5). Overall, 67 patients (13.5%) in the pembrolizumab group and 99 patients (19.9%) in the placebo group received ≥1 line of subsequent anticancer drug therapy. Of patients who received ≥1 line of subsequent drug therapy, most in the pembrolizumab group (90.0%) and placebo group (85.9%) received a VEGF/VEGFR-targeted therapy. Furthermore, 23.9% of patients in the pembrolizumab group and 59.6% in the placebo group received an anti–PD-1/PD-L1 agent. There were 77 TFST events observed in the pembrolizumab group and 110 in the placebo group. Compared with placebo, adjuvant treatment with pembrolizumab delayed TFST (HR 0.67, 95% CI 0.50-0.90; medians not reached):
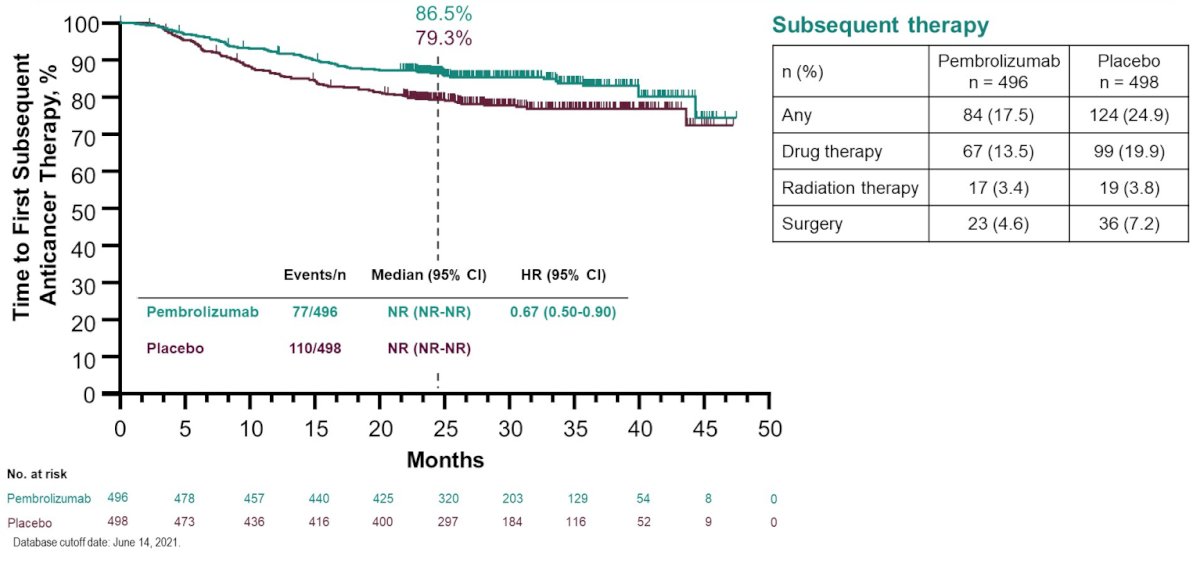
A total of 108 PFS2 events were observed, 40 (8.1%; 12 death events and 28 progression events) in the pembrolizumab group and 68 (13.7%; 14 death events and 54 progression events) in the placebo group. PFS2 was also delayed with pembrolizumab compared with placebo (HR 0.57, 95% CI 0.39-0.85; medians not reached):

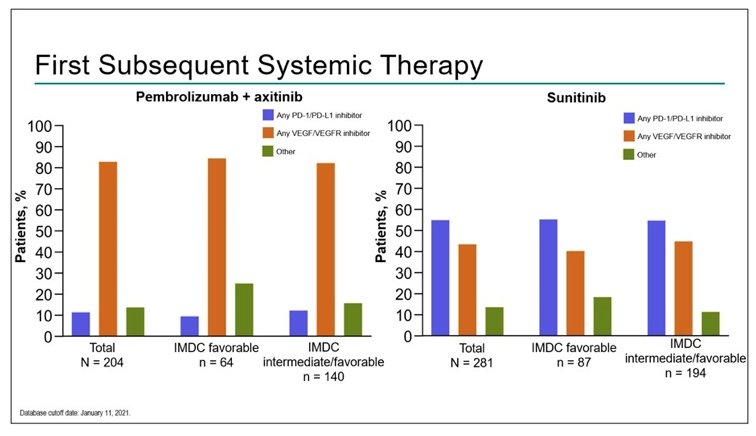
Dr. Suarez then discussed the KEYNOTE-426 trial, which also assessed progression after first subsequent therapy for patients treated with pembrolizumab + axitinib versus sunitinib for first-line mRCC patients. Median time from randomization to the database cutoff date (January 11, 2021) was 42.8 months (range, 35.6-50.6). Overall, 47.2% of patients (204/432) in the pembrolizumab + axitinib arm and 65.5% of patients (281/429) in the sunitinib arm received ≥1 line of subsequent anticancer therapy. For patients who received subsequent therapy, anti–PD-1/PD-L1 agents were the first subsequent treatment for 11.3% of patients (23/204) in the pembrolizumab + axitinib arm and 54.8% of patients (154/281) in the sunitinib arm. In the pembrolizumab + axitinib arm, 82.8% of patients (169/204) received a VEGF/VEGFR inhibitor as first subsequent therapy, as did 43.4% (122/281) in the sunitinib arm. The following figure summarizes first-subsequent anticancer therapy:
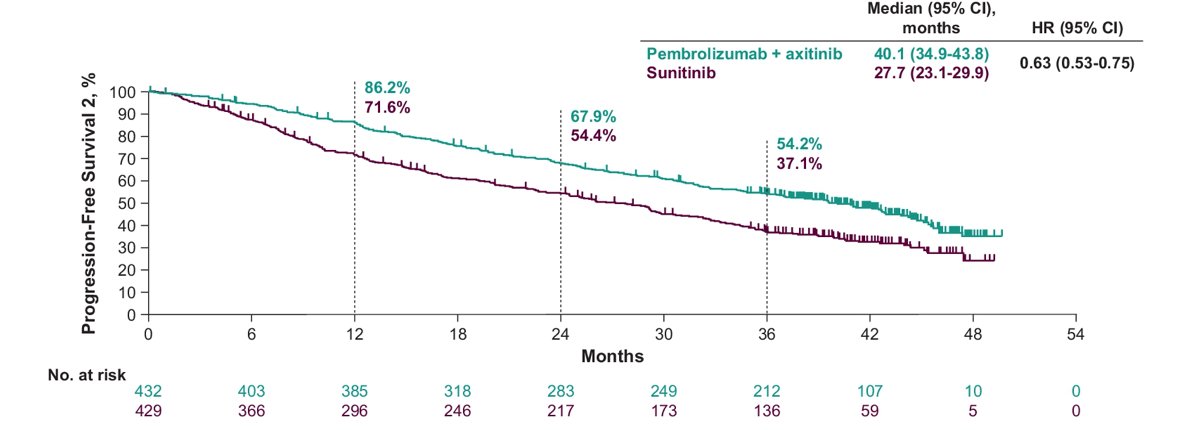
In the ITT population, the median time to PFS2 was 40.1 months for pembrolizumab + axitinib versus 27.7 months for sunitinib (HR 0.63, 95% CI 0.53-0.75):
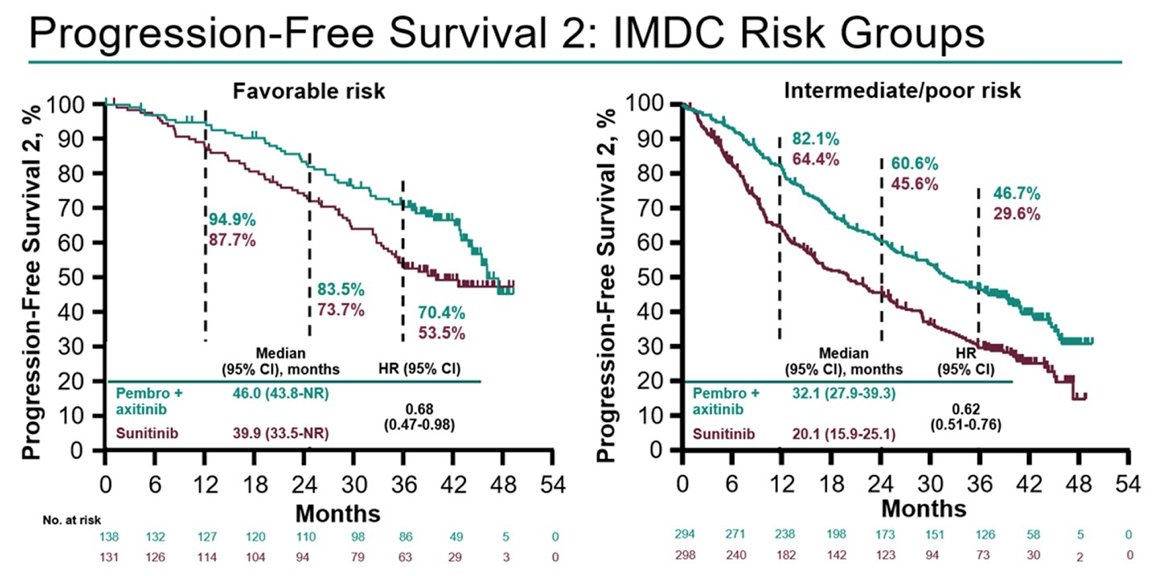
Among patients with IMDC favorable risk disease, the median time to PFS2 was 46.0 months for pembrolizumab + axitinib versus 39.9 months for sunitinib (HR 0.68, 95% CI 0.47-0.98). Among patients with IMDC intermediate/poor risk disease, the median time to PFS2 was 32.1 months for pembrolizumab + axitinib versus 20.1 months for sunitinib (HR 0.62, 95% CI 0.51-0.76):
The final abstract discussed by Dr. Suarez was the impact of subsequent therapies in patients with advanced RCC receiving lenvatinib + pembrolizumab in the CLEAR trial. PFS2 was evaluated in all pts randomly assigned to lenvatinib 20 mg orally QD + pembrolizumab 200 mg IV Q3W (n=355) or sunitinib 50 mg orally QD (4 weeks on/2 weeks off) (n=357) using Kaplan-Meier estimates, and compared between treatment arms via a log-rank test stratified by geographic region and MSKCC prognostic groups. Among patients who received subsequent anticancer therapy in the lenvatinib + pembrolizumab (n=117 patients) and sunitinib (n=206 patients) arms, median time to next-line therapy of therapy was 12.2 months (range 1.45–37.36) and 6.4 months (range 0.39–28.52), respectively. A summary of subsequent therapies is as follows:

Median duration of first subsequent anticancer therapy was 5.2 months (range 0.10–30.23) in the lenvatinib + pembrolizumab arm and 6.8 months (range 0.03–30.72) in the sunitinib arm. Among all patients, PFS2 was longer with lenvatinib + pembrolizumab than with sunitinib (median not reached vs 28.7 months; HR 0.50, 95% CI 0.39–0.65):

Dr. Suarez highlighted the following considerations when considering sequencing challenges:
- With the advent of first-line IO-based combinations, the therapeutic sequence in mRCC has changed
- No prospective randomized studies exploring what to do after failure on IO-based combinations in front-line are available (several are ongoing)
- After IO-IO, patients are progressing to “one mechanism of action”
- After IO-VEGF TKI patients are progressing to “two mechanisms of action”
- Guidelines recommend in second line a TKI that has not been used before
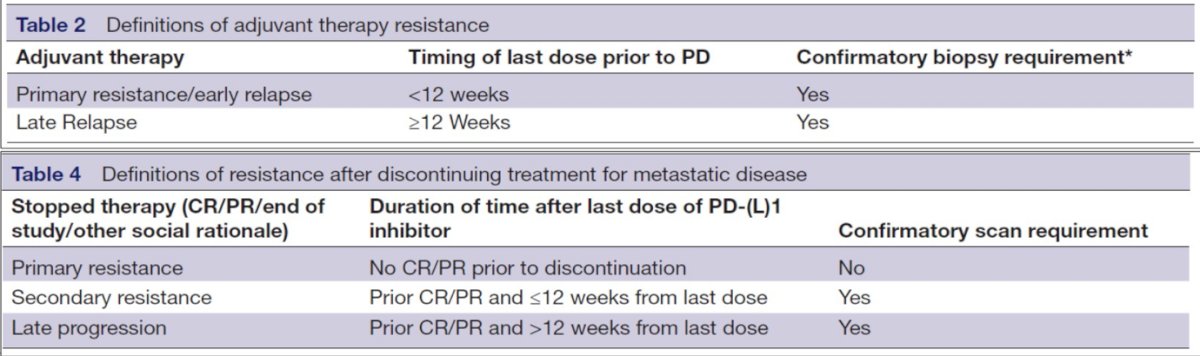
- At what point from the time a patient completes immunotherapy (adjuvant or metastatic) until progression, should we consider the patient “immunotherapy resistant”? As follows are the recommendations from the first meeting of the SITC Immunotherapy Resistance Taskforce for defining tumor resistance to PD-1 pathway blockade:
According to Dr. Suarez, there are four options with regards to what to do next after progression on first-line therapy, including: (i) VEGF-targeted monotherapy, (ii) IO after IO, (iii) new targets, and (iv) ongoing trials. With regards to VEGF-targeted monotherapy, there are retrospective studies that have tested TKI after IO, with ORRs ranging from 20-54% and PFS or TTF ranging from 6-13 months, as summarized in the following table:

Additionally, there are prospective trials that have tested TKI after IO, with ORRs ranging from 19-45% and PFS ranging from 5.6-9.3 months, as summarized in the following table:

For IO after IO, this may be thought of as an IO “boost/rechallenge”, which has been tested in the HCRN-GU-16-260, TITAN-RCC, OMNIVORE-RCC, and FRACTION trials:

In a meta-analysis assessing salvage nivolumab + ipilimumab, this analysis included 7 studies (retrospective and prospective), with 310 patients and an ORR of 14%.
With regards to new targets, Dr. Suarez highlighted work presented by Dr. McDermott at ESMO 2021 testing belzutifan + cabozantinib. The ORR in this study was 28.8% (all confirmed partial responses) and durable control rate was 92.3%, with no appreciable differences across IMDC risk category and prior anticancer therapy:
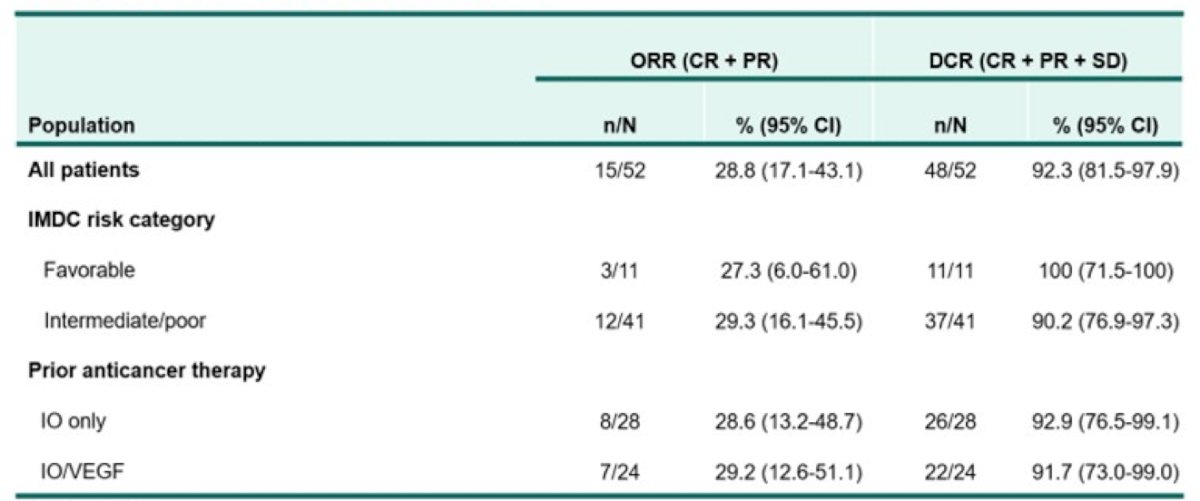
Overall, 86.5% of patients had a reduction in target lesion size. Median duration of response was not reached (range, 3.7+ to 14.8+ months) and all responses were ongoing as of the data cutoff date. Median PFS was 16.8 months (95% CI, 9.2 to not reached), and PFS rate at 12 months was 65%; OS rate at 12 months was 81%.
Telaglenastat has also been tested in the ENTRATA (with everolimus) and CANTATA (with cabozantinib) clinical trials. The telaglenastat + everolimus combination had a 2.2% partial response rate and a 56.5% stable disease rate, whereas telaglenastat + cabozantinib had an overall response rate of 31.2%, including 0.9% complete response, 30.3% partial response, and 54.8% stable disease.
There are several ongoing clinical trials in this disease space, including:
- MK-6482-005 (phase III): belzutifan vs everolimus, with a primary endpoint of overall survival and PFS (n = 736)
- MK-6482-011 (phase III): belzutifan + lenvatinib vs cabozantinib, with a primary endpoint of overall survival and PFS (n = 708)
- CONTACT-03 (phase III): atezolizumab + cabozantinib vs cabozantinib, with a primary endpoint of overall survival and PFS (n = 500)
- TINIVO-2 (phase III): nivolumab + tivozanib vs tivozanib, with a primary endpoint of PFS (n = 326)
Dr. Suarez concluded her presentation by discussing sequencing challenges in RCC with the following take-home messages:
- Results from the analysis of PFS2 in first-line IO combination trials support the long-term benefit of these combinations for first-line treatment of patients with advanced RCC
- With the advent of first-line combinations, the second-line scenario has changed and data from phase 3 studies are awaited
- After IO/IO or IO/TKI, a TKI has the strongest evidence as of now
- Ongoing trials may provide more information about maintenance IO after IO
Presented by: Cristina Suarez, Hospital Universitari Vall d’Hebron – Vall d’Hebron Institute of Oncology, Barcelona, Spain
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.


