Prostate cancer patients with post-radical prostatectomy biochemical recurrent (PSA > 0.5ng/mL; > 0.2ng/mL if within 12 months of radical prostatectomy) and no metastases on conventional imaging who are candidates for standard of care salvage therapy (radiotherapy to prostate bed and pelvic nodes with short-term androgen deprivation therapy) are eligible. Prior to study registration, patients must undergo standard of care baseline PET (18F-fluciclovine but PSMA radiotracers permitted pending commercial availability). Based on institutional clinical interpretation of the standard of care PET, patients will be placed in Cohort 1 (PET-negative) or 2 (PET-positive for extra-pelvic metastases). Cohort 1 will be randomized to standard of care salvage therapy +/- apalutamide for 6 months and Cohort 2 will be randomized to standard of care salvage therapy and apalutamide +/- metastasis-directed radiotherapy to PET-positive lesions.
The trial schema for INDICATE is as follows:
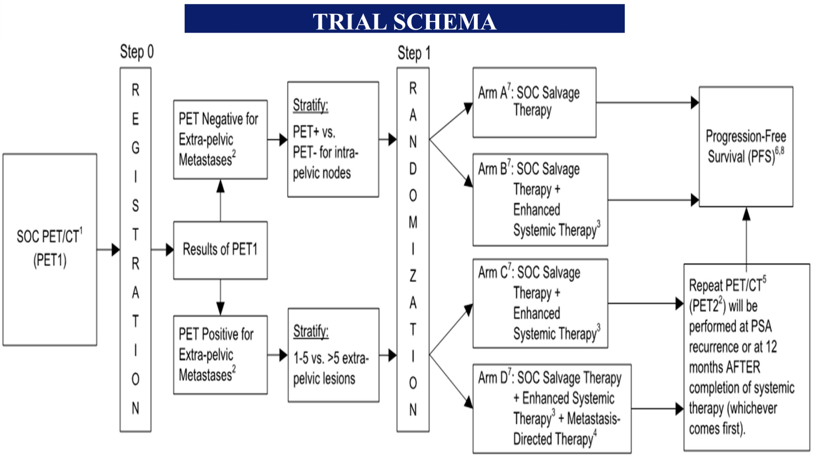
The primary endpoint is PFS, defined as time from randomization to radiographic progression on conventional imaging, symptomatic disease or death. Primary objectives are to evaluate whether addition of apalutamide to standard of care salvage therapy and addition of metastasis-directed radiotherapy to standard of care salvage therapy and apalutamide could prolong PFS in Cohorts 1 and 2, respectively. For Cohort 1, 480 patients will be randomized with 85% power to distinguish 5-year PFS rate of 90% (apalutamide arm) versus 80% (standard of care arm) using one-sided stratified log-rank test with type I error of 0.025. For Cohort 2, 324 patients will be randomized with 85% power to distinguish 5-year PFS rate of 76.5% in the experimental arm from 61.5% in the control arm. Secondary endpoints include overall and event-free survival, toxicity, and PET progression. This trial is currently actively recruiting patients.
Clinical trial information: NCT04423211
Presented by: Neha Vapiwala, MD, FACR, Professor and Vice-Chair of Education in the Department of Radiation Oncology at University of Pennsylvania, Philadelphia, PA
Co-Authors: Yu-Hui Chen, Steve Y. Cho, Fenghai Duan, Christos Kyriakopoulos, Daniel H. Shevrin, Rana R. McKay, Bridget F. Koontz, Evan Y. Yu, Volkan Beylergil, David A. Mankoff, Jonathan McConathy, Glenn Liu, Terence Z. Wong, Michael Anthony Carducci; University of Pennsylvania, Philadelphia, PA; Dana-Farber Cancer Institute, Boston, MA; University of Wisconsin SMPH, Department of Radiology, University of Wisconsin Carbone Cancer Center, Madison, WI; Brown University, Providence, RI; University of Wisconsin Carbone Cancer Center, Madison, WI; NorthShore University Health System, Evanston, IL; University of California San Diego, Moores Cancer Center, La Jolla, CA; Duke University Medical Center, Durham, NC; Division of Oncology, Department of Medicine, University of Washington, Seattle, WA; Columbia, New York, NY; University of Alabama at Birmingham, Birmingham, AL; Chief, Division of Nuclear Medicine and Radiotheranostics Professor of Radiology Professor in Medicine, Division of Medical Oncology Duke Cancer Institute Medical Physics Graduate Program Duke University Medical Center, Durham, NC; Sidney Kimmel Cancer Center, Johns Hopkins University School of Medicine, Baltimore, MD
Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


