As such, sunitinib has remained the National Comprehensive Cancer Network (NCCN) guideline-recommended first-line treatment. Initial results of the CALYPSO trial suggested encouraging progression-free survival (PFS) and overall survival (OS) for patients with advanced papillary RCC treated with durvalumab and savolitinib. In the Kidney and Bladder Poster Discussion session at the 2021 American Society of Clinical Oncology ASCO) Annual Meeting, Dr. Cristina Suarez-Rodriguez presented the results of a study examining durvalumab and savolitinib in patients with MET-driven, metastatic papillary renal cancer (PRC). Savolitinib is a potent and selective MET inhibitor with activity in MET-driven papillary renal carcinoma (PRC) while the PD-L1 inhibitor durvalumab may potentiate its effect.
The authors performed a single-arm phase I/II trial assessing the combination of durvalumab (1500mg every 4 weeks) and savolitinib (600mg daily) together in metastatic PRC, following a 4-week savolitinib run in (NCT02819596). In a pre-planned analysis, the authors examined a biomarker-driven assessment of treatment response, with this analysis focusing on patients with MET DNA alterations (central analysis of chromosome 7 gain, MET or hepatocyte growth factor (HGF) amplification, or MET kinase domain mutations). The authors report confirmed response rate (RR) (RECIST v1.1), progression-free survival (PFS), tolerability (CTCAE v4.03), and overall survival (OS).
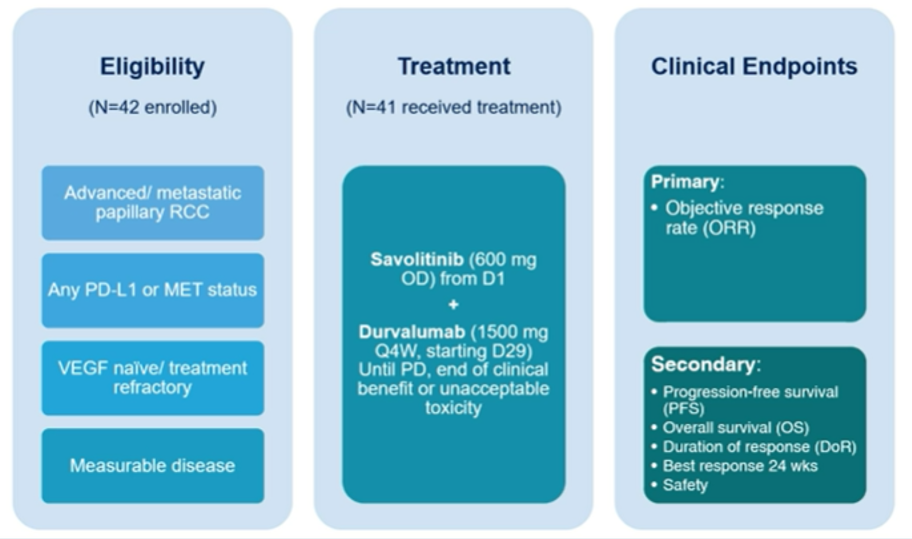
The authors enrolled 42 patients with metastatic PRC, of whom 41 received treatment. Over a median follow-up of 26.8 months, the confirmed RR was 29% (12/41) and median PFS was 4.9 months (95% CI 2.5-10.0).

In the biomarker-stratified analysis, 14 patients (34%) were identified as having MET-driven disease. In this group, 71% (10/14) had not previously received systemic therapy and 7% (1/14) were PD-L1 positive. In terms of risk stratification, IMDC good, intermediate, and poor-risk disease occurred in 36% (5/14), 57% (8/14), and 7% (1/14) of patients in this subset, respectively. Over a median follow-up of 26.8 months, among patients with MET-driven tumors, the confirmed RR was 57% (8/14) with a duration of response at 9.4 months (95% CI 3.9-Not reached [NR]).
Further, median PFS and OS in these patients with MET-driven tumors were 10.5 months (95% CI 2.9-15.7) and 27.4 months (95% CI 7.3-NR), respectively. PFS was substantially longer for patients with MET-driven than non-MET-driven tumors.
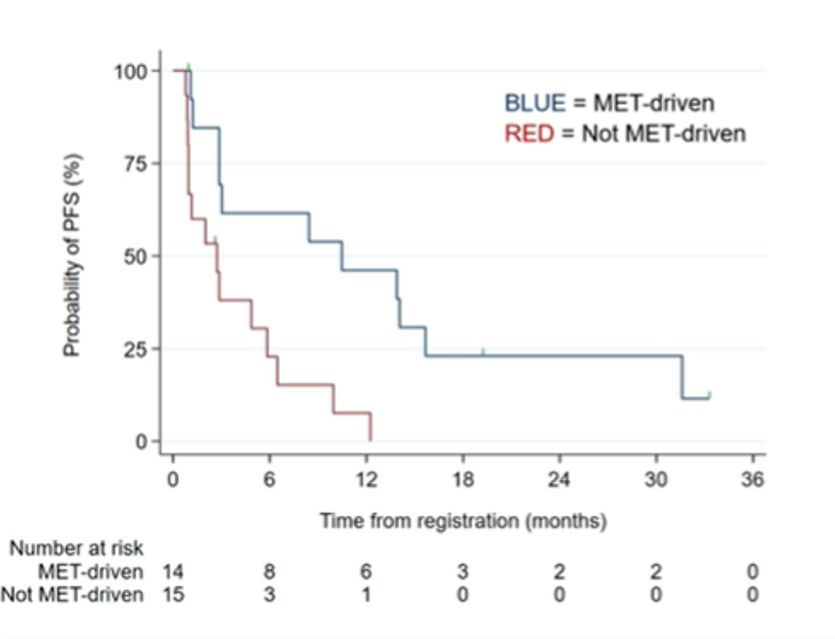
The authors concluded that the combination of savolitinib and durvalumab has encouraging clinical activity in patients with MET-driven PRC and, based on these data, a randomized phase III study is planned.
Presented by: Cristina Suarez-Rodriguez, Department of Oncology, Hospital Vall d’Hebron, Barcelona, Spain
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021