As a rare surgical clinical trial, it has gained much attention. In this study, the authors assessed the role of neoadjuvant chemohormonal therapy prior to radical prostatectomy (RP) in men with clinically localized high-risk disease. The rationale for the study included the fact that men with clinically localized high-risk prostate cancer have a significant risk of biochemical recurrence (BCR) after radical prostatectomy. Neoadjuvant androgen deprivation therapy (ADT) and radical prostatectomy have not improved outcomes compared to radical prostatectomy alone. However, at the time, there was evidence that docetaxel chemotherapy prolongs survival in castrate-resistant metastatic prostate cancer, it made sense to assess it in an earlier cohort – indeed since that time, docetaxel has made its way up to the treatment of high volume metastatic hormone-sensitive prostate cancer.
In this study, all men had clinical stage T1-3a disease by digital rectal examination. PSA had to be lower than 100 ng/ml, and all were diagnosed with adenocarcinoma of the prostate, without any radiographic evidence of metastasis. Furthermore, all men had either a biopsy Gleason score of 8-10 or a Kattan preoperative nomogram probability of <60% biochemical progression-free survival at five years after radical prostatectomy.
The study design is shown in Figure 1 below:

The patient baseline characteristics in both arms are shown in Table 1 below:

It is noteworthy, that approximately 40% of the men received salvage radiation therapy, ADT, or both, before meeting the definition of BCR. This plays an important role in the consideration of the results.
The summary of outcomes are shown in the table below:

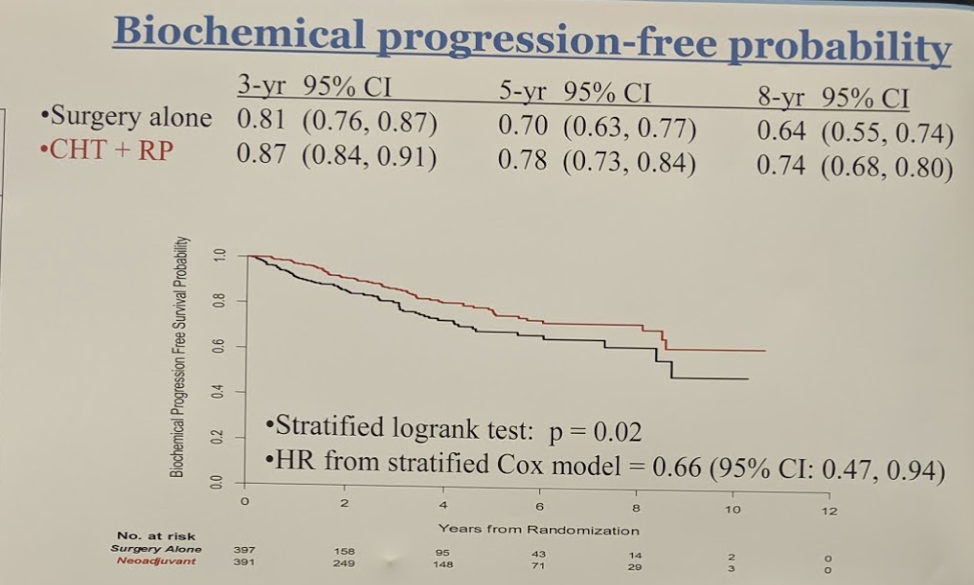
More importantly, however, it did demonstrate significant freedom from treatment failure is shown in figure 2, again showing an advantage to the neoadjuvant arm, and lastly, figure 3 demonstrates the overall survival rate in both treatment arms.
Figure 2 - Freedom from treatment failure (receipt of radiation or hormones):
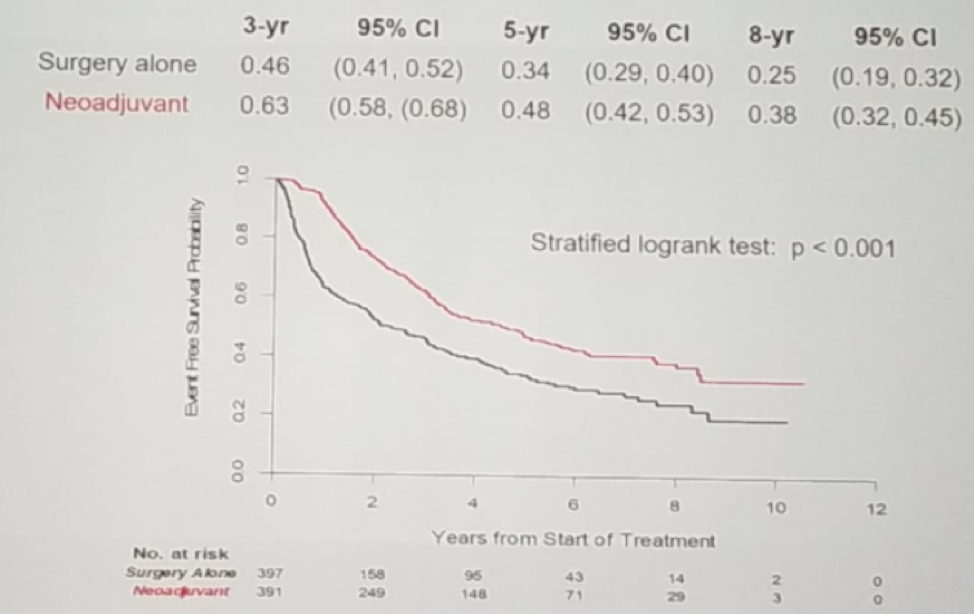
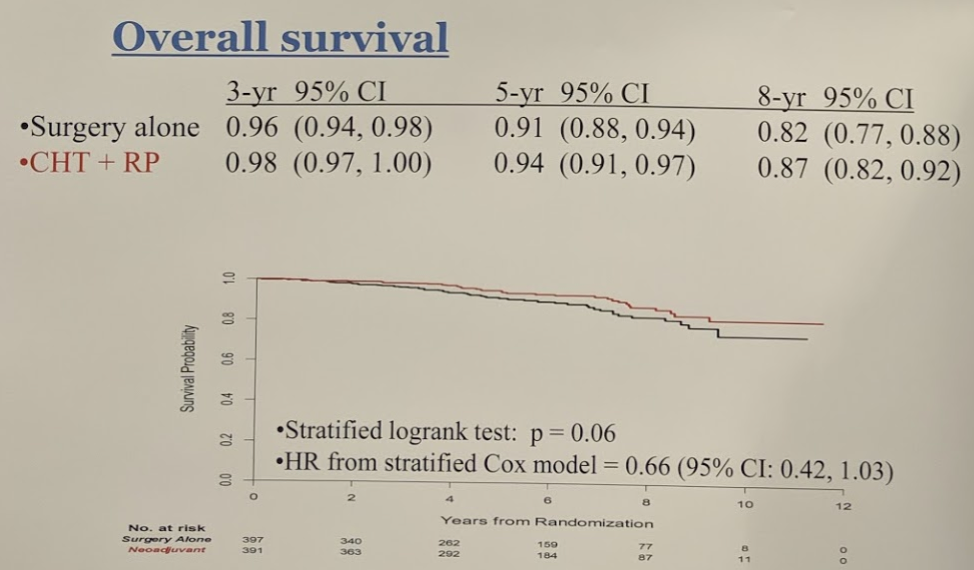
Though it did meet its primary endpoint of BCR-free survival at 3 years, which was maintained through 8 years, it has not translated to an OS benefit at 8 years. These data may support the use of neoadjuvant chemohormonal therapy before radical prostatectomy, for at least delaying biochemical progression-free survival, in men with clinically localized high-risk prostate cancer.
In a candid discussion with Dr. Eastham, he noted the following:
- The BCR-free survival was confounded by the fact that, as this study was developed 17 years ago, they did not account for the early salvage XRT that was going to be administered. So, patients often got salvage XRT, which affected the definition of BCR.
- More importantly, in the subset of patients that did not get any subsequent treatment for BCR, they noted that neoadjuvant therapy with docetaxel and hormones had a significant impact
- However, this is weighed against the toxicity of therapy
This study only reports initial pathology reports from institutions. A centralized review is pending. Dr. Eastham, unfortunately, could not comment on intra-operative toxicity and complications but did not that anecdotally, there was “treatment-effect” that many surgeons noted – and may make for a more complicated surgery.
Clinical trial information: NCT00430183
Presented by: James Andrew Eastham, Memorial Sloan Kettering Cancer Center, New York, NY
Co-authors: Glenn Heller, Susan Halabi, Paul Monk, Steven K. Clinton, Russell Zelig Szmulewitz, Jonathan Coleman, Martin Gleave, Christopher P. Evans, David W. Hillman, Himisha Beltran, Mary-Ellen Taplin, Olwen Mary Hahn, J Kellogg Parsons, Eric Jay Small, James Mohler, Michael J. Morris
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, @tchandra_uromd, @JEFFUrology at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA