
As response rates are moderate at best, identifying alternative systemic therapies for patients that are not likely to benefit from ICI’s are critical.
FGFR3 alterations have been shown preclinically to be oncogenic drivers of bladder cancer. FGFR3 inhibitors are revolutionizing the clinical management of metastatic urothelial carcinoma in biomarker-selected and potentially poor immunoncology responding patients. We know that 20% of patients with mUC harbor FGFR3 mutations or fusions (M/F), which may result in lower sensitivity to ICI. Targeting FGFR3, therefore, may be an important alternative option for patients that are likely not going to benefit from ICIs. Vofatamab (B-701, V) is a fully human monoclonal antibody against FGFR3 that blocks activation of both the wildtype and genetically activated receptor. A diagram and explanation of its mechanism is below:
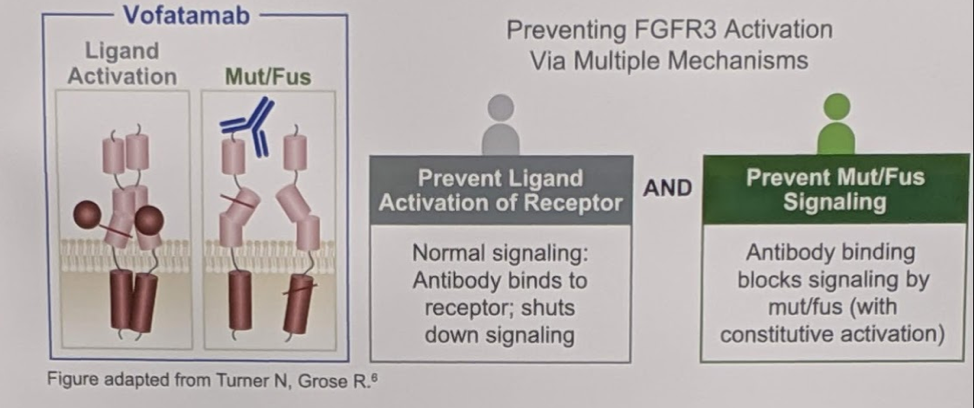
As response rates are moderate at best, identifying alternative systemic therapies for patients that are not likely to benefit from ICI’s are critical.
This drug is being tested in combination with other established agents. FIERCE-21 was a phase 1b/2 study designed to evaluate vofatamab monotherapy and combination with docetaxel. The results of this study were presented by Dr. Necchi at GU ASCO 2019. The median progression-free survival (PFS) was not reached in the combination arm, and it was four months in the monotherapy arm. Vofatamab was shown to be well-tolerated and had no long-term safety issues, allowing patients to receive long term treatment.
In this abstract, the authors assessed a combination of V with Pembrolizumab (VP) in a Phase 1b/2 study. The study design is below:

There is a 2-week lead-in with V monotherapy. Patients are stratified by FGFR3 status – 26 patients in each arm initially, but expanded with an additional 22 patients. Two interim analyses were planned – the first one after 26 patients. This is the first interim analysis.
They enrolled mUC patients with failure to ≥ 1 prior line of chemotherapy or recurrence ≤ 12 months of (neo)adjuvant chemotherapy, measurable disease and ECOG <2. Treatment consisted of V at 25 mg/kg alone for 2 weeks of monotherapy. This was then followed by combination with Pembrolizumab 200 mg every 3 weeks. During the V monotherapy window, paired tumor biopsy was obtained.
Efficacy was assessed by investigators (RECIST 1.1). Primary objectives were safety and activity, specifically objective response rate (ORR)
To date, 28 patients have received treatment (Mut/Fusion: 8, WT:20, M/F+: 7). Full demographics are below:

WT patients were unselected for PD-1 status. The cohort was predominately male (61%) white (96%), all had received at least 1 line of prior chemo and 64% had Bellmunt scores of ≥1.
At the time of the reporting, 7 (35%) of the WT and 2 (25%) of the mut/fus patients remained on treatment. The majority of patients received 8 doses of V.
When looking at safety, the safety profile is consistent with previously reported data for pembrolizumab. As indicated before, Vofatamab monotherapy is well tolerated. More of the adverse events came from the pembrolizumab. TEAE occurring in >20% of patients were nausea, anemia, diarrhea, and fatigue. AE profile is shown below:

In terms of oncologic outcomes, in 22 patients with evaluable lesions, the ORR was 36% (8/22) – 33% in the WT cohort and 43% in the mut/fus cohort. Responses occurred at a median of 3.5 months, similar to the Fierce-21 study. At 5+ months the median PFS has not been reached.
An interesting separate analysis, they looked at response related to the five established molecular subtypes and some important trends are noted:
As seen below, the responses were similar regardless of FGFR3 status:
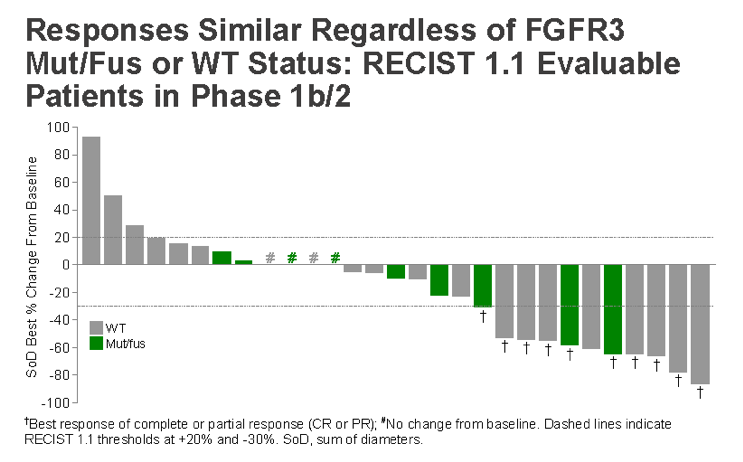
An interesting separate analysis, they looked at response related to the five established molecular subtypes and some important trends are noted:
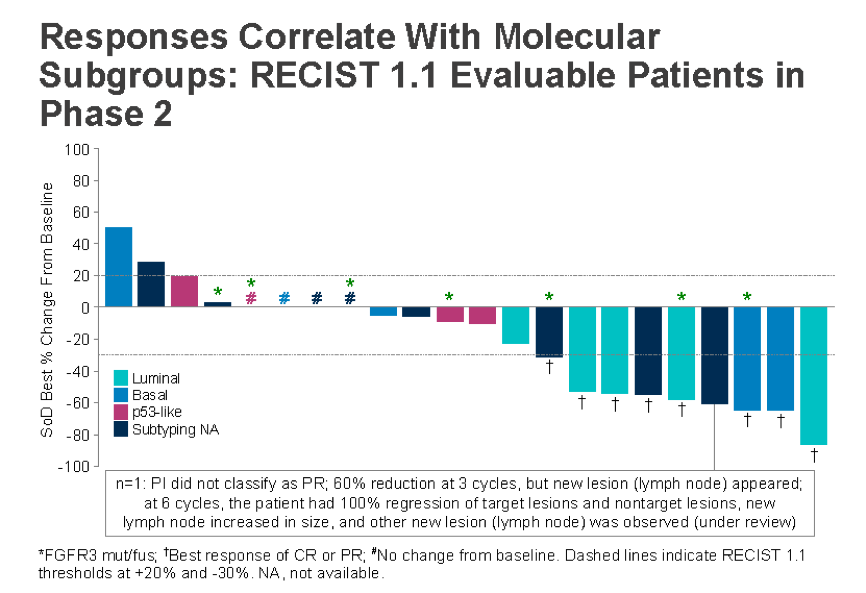
The best responses are in those patients with luminal or basal subtype, or patients with no subtyped identified. P53-like patients did not respond nearly as well. This is further seen below in the duration of response separated by molecular subtype:

They did note that they were able to safely get paired biopsies before and after the V monotherapy. Below, they found that the lead in V monotherapy induced some important genomic changes:
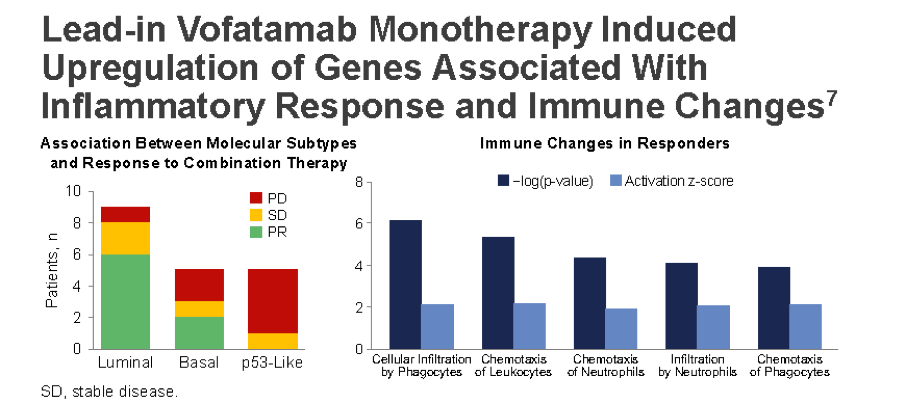
Specifically in genes associated with an inflammatory response.
Based on data to date, the VP combination appears to be well tolerated with an encouraging ORR and prolonged PFS in the WT cohort – a response greater than one would anticipate from Pembrolizumab alone based upon historical data.
Presented by: Arlene O. Siefker-Radtke, MD, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, MD Anderson Cancer Center, University of Texas, Houston, TX
Co-Authors: Graeme Currie, Esteban Abella, Daniel A. Vaena, Arash Rezazadeh Kalebasty, Giuseppe Curigliano, Krzysztof Tupikowski, Zoran Gojko Andric, Iwona Lugowska, William Kevin Kelly; Rainier Therapeutics Inc, San Leandro, CA; Rainier Therapeutics, Inc, San Leandro, CA; University of Iowa Hospitals and Clinics, Holden Comprehensive Cancer Center, Iowa City, IA; Norton Cancer Institute, Louisville, KY; University of Milano, European Institute of Oncology, Division of Early Drug Development, Milan, Italy; Dolnoslaskie Centrum Onkologii, Dolnoslaskie, Poland; Clinical Hospital Center, Bezanijska Kosa, Belgrade, Serbia; Centrum Onkologii - Instytut im. Marii Sklodowskiej - Curie, Warsaw, Poland; Sidney Kimmel Cancer Center at Thomas Jefferson University, Philadelphia, PA; Sidney Kimmel Cancer Center at Thomas Jefferson University, Philadelphia, PA
Written by: Thenappan Chandrasekar, MD, Clinical Instructor, Thomas Jefferson University, @tchandra_uromd, @JEFFUrology at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA